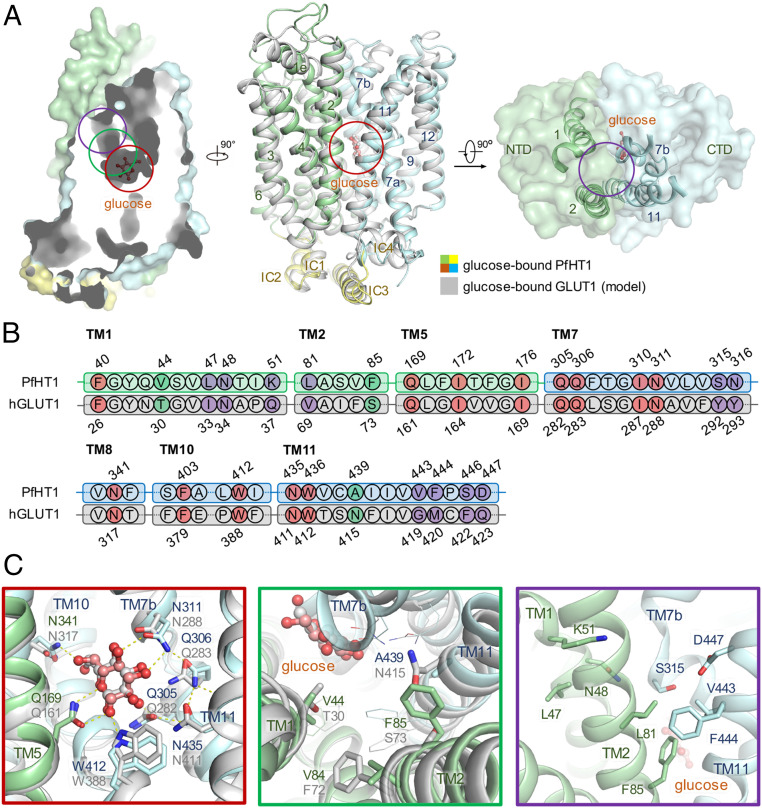
Fig. 1.
Structural comparison between PfHT1 and hGLUT1 reveals potential druggable site for PfHT1-specific inhibitors. (A) Superimposition of structures between occluded glucose–PfHT1 complex (domain colored, PDB ID code 6M20) and a model of the outward-occluded glucose–hGLUT1 complex (gray). The protein structures are shown in cartoon representation. The amino-terminal (NTD), carboxyl-terminal (CTD), and intracellular helical (ICH) domains of PfHT1 are colored in pale green, pale cyan, and yellow, respectively. (B) Sequence alignment of PfHT1 (green and cyan) and hGLUT1 (gray) highlights the portion that engages with glucose. The residues involved in the glucose-binding site, the allosteric pocket, and the connecting channel are colored in red, purple, and green, respectively. Residue numbers for PfHT1 and hGLUT1 are shown above and below the alignment, respectively. (C) Close-up views of the glucose-binding site, connecting channel, and the extended pocket are presented below, with red, green, and purple boxes, respectively.