Abstract
Background: Depression is a common complication of pregnancy and vitamin D deficiency is one biological risk factor for postpartum depression (PPD).
Materials and Methods: We evaluated the ratio of 24,25(OH)2D and 25(OH)D serum concentrations referred to as the Vitamin D Metabolite Ratio (VMR), a new candidate biomarker during pregnancyand its relationship with PPD. Women were enrolled in the first trimester of pregnancy and followed through four timepoints.
Results: A total of 89 women had complete depression, biomarker and demographic data and 34% were at risk for PPD (CES-D≥16). Stepwise multiple logistic regression models for PPD risk were carried out with eight predictors. Results showed that only lower VMR, OR = 1.43, 95% CI 1.10–1.86, p = 0.007, and Hispanic/Latina identification, OR = 3.83, 95% CI 1.44–10.92, p = 0.007 were significantly associated with higher PPD risk.
Conclusion: Routine prenatal screening for vitamin D metabolites, particularly in Hispanic/Latina women, may identify women at risk for PPD.
Keywords: vitamin D, deficiency, postpartum depression, pregnancy
Introduction
Postpartum depression (PPD) is a severe subtype of depression occurring after delivery, which interferes with daily functioning and requires treatment. PPD prevalence rates in the United States range from 13% to 19%1 and the rate varies by region, population, and how PPD is assessed. In addition, rates are higher in ethnic and racial minorities (35%–67%).2–4 In fact, 49% of black mothers5,6 and 54% of Latinas experienced depressive symptoms after delivery.7,8 One of the biggest risk factors for PPD is depression during pregnancy.9 Prenatal depression is quite common with rates ranging between 12% and 22%, which is double the rate in the general female population10–13 and has been associated with poor maternal health behaviors14 and risk of PPD.15 Furthermore, prenatal depression and anxiety are associated with increased risk for other adverse birth outcomes, including preterm birth (PTB), low birth weight, and intrauterine growth restriction,16–19 although more research is needed to differentiate unique predictive effects on outcomes.20
Literature from the past decade supports the association between low levels of circulating 25-hydroxyvitamin D (25(OH)D) and PPD.21 In 2010, the first meta-analysis reported that circulating vitamin D was inversely associated with depression in 31,424 males and females; however, none of the studies included pregnant or postpartum women.22 Three prospective studies have since reported that low levels of prenatal vitamin D were associated with later PPD symptoms.23–25 Two of the three reported a significant association between low prenatal vitamin D levels and PPD symptoms.23,25 Although the third study did not find a significant association between 25(OH)D levels and PPD, their study was limited by registry data on antidepressant use as a proxy for PPD; they did not require a medical diagnosis of PPD or use any validated depression screening survey.24
Five recent cross-sectional studies reported that prenatal depressive symptoms were associated with low prenatal vitamin D levels.26–30 Finally, two published studies have reported that PPD symptoms have been associated with low postpartum vitamin D levels.31,32 Most recently, a systematic review and meta-analysis of nine longitudinal studies with 8,470 participants found that serum 25(OH)D levels <50 nmol/L was associated with increased risk of PPD compared to those with 25(OH)D levels ≥50 nmol/L (odds ratio [OR] 3.67; 95% CI 1.72–7.85). They did not, however, find a significant association between low prenatal vitamin D levels and prenatal depressive symptoms.33
Vitamin D3, also known as cholecalciferol, is created by skin cells called keratinocytes in response to UVB light and can also be obtained from a few food sources, such as fatty fish like salmon, and during pregnancy by prenatal vitamins. These forms of vitamin D do not have any biological activity in the body and must be modified in the liver to generate 25(OH)D. This 25(OH)D is the major form of vitamin D circulating in the bloodstream, and when needed to function, the body requires a final metabolic conversion to the biologically active form. For example, the synthesis and function of naturally occurring defensin molecules against bacterial antigens are regulated in the cell by 25-hydroxylated metabolites of vitamin.34
The active form of vitamin D, 1,25(OH)2D3 has also been found to inhibit antigen-induced T cell proliferation and cytokine production.35 While vitamin D deficiency could lead to lowered immunity in general, it could also lead to the pathogenesis of autoimmune diseases because vitamin D receptor (VDR) actions affect inflammation, autophagy, and microbiota composition. Indeed, vitamin D affects many immune cell types and its deficiency has been associated with several diseases such as cancer, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, and depression.34,36,37
Astounding rates of poor vitamin D status are found among pregnant women and deficiency rates are higher in women with darker skin.35,38–40 Seventy-five percent of black pregnant women and fifty percent of white pregnant women living in a northeastern United States city had mid-pregnancy serum 25(OH)D concentrations <32 ng/mL,41 the prenatal level considered “optimal” by experts.40,41 Black women consistently have lower total 25(OH)D than white women and frequently meet criteria for vitamin D insufficiency (i.e., 25(OH)D <20 ng/mL [<48.4 nmol/L]),41–43 particularly in pregnancy.44,45 Paradoxically, black women have higher bone mineral density, but still have a lower risk of osteoporosis and resulting fragility fractures compared to white women.46–49 Interestingly, it was reported that black Americans have significantly lower mean total 25(OH)D concentrations compared to white Americans; however, their concentrations of free or bioavailable 25(OH)D may be equivalent.50,51
Robust assays to directly measure bioavailable 25(OH)D are not yet available, and therefore alternative methods to evaluate vitamin D status are needed. Recent evidence suggests that vitamin D sufficiency may be reflected by concentrations of serum 24,25(OH)2D,52–54 and the major product of catabolism of 25(OH)D is 24,25-dihydroxyvitamin D3 (24,25(OH)2D). Enzymatic synthesis of 24,25(OH)2D is proportional to 25(OH)D substrate concentrations. Furthermore, concentrations of both metabolites in circulation are highly correlated.55 In addition, expression of the 24-hydroxylase enzyme (CYP24A1) that converts 25(OH)D to 24,25(OH)2D is regulated, in part, by VDR activity.56,57 Therefore, concentrations of 24,25(OH)2D may be an even better indicator of vitamin D sufficiency than 25(OH)D itself because production of 24,25(OH)2D relies upon both concentrations of 25(OH)D and vitamin D-regulated expression of CYP24A1. Recent findings also suggest that vitamin D sufficiency may be reflected by the ratio of 24,25(OH)2D and 25(OH)D serum concentrations, referred to as the Vitamin D Metabolite Ratio (VMR).52–54
To our knowledge, no study has investigated the association of depressive symptoms and lower vitamin D status as measured by the VMR, particularly in the context of maternal health. This secondary data analysis sought to determine whether lower vitamin D status predicted risk for postpartum depression (PPD) in a racially diverse sample of women from the Behavior In Pregnancy Study (BIPS). We hypothesized that lower vitamin D status, as measured by the VMR, would predict risk for PPD, regardless of race.
Materials and Methods
Participants
This secondary data analysis was conducted with a subset of sample from the BIPS, which enrolled women in the first trimester of pregnancy and followed them into the postpartum period with interviews, ultrasound, and biomarkers, including vitamin D metabolites. Participant recruitment and data collection occurred in prenatal clinics and private practice locations across Los Angeles, California. Informed consent was obtained from all participants included in the study.58,59 IRB approval for this study was received from Cedars-Sinai Medical Center. The BIPS recruited women who gave birth to liveborn infants, were English or Spanish speaking, had a singleton intrauterine pregnancy, and were under 20 weeks gestation at the time of recruitment. Women in BIPS who had vitamin D data for at least one time point during pregnancy as well as demographic and data on prenatal or postpartum depressive symptoms (N = 160) were included. Only 89 of these 160 women completed the PPD screening. Compared to participants in the larger sample, women in this subsample were less likely to be black (29% vs. 47%). This subsample did not differ significantly from the larger sample on any other variable, including body mass index (BMI), age, prenatal or postpartum vitamin D metabolites (vitamin D2, vitamin D3, and VMR), smoking in pregnancy, marital status, education, or prenatal depression symptoms (Beck Depression Inventory [BDI]).
Study variables
Demographic information included maternal age, maternal weight and height, education, marital status, and race-ethnicity. Medical variables included fetal sex and prenatal infections, including urinary infections, asymptomatic bacteria, genital tract infection, colonization with group B streptococcus, HIV, chlamydia, gonorrhea, syphilis, Trichomonas, candidiasis, and bacterial vaginosis, including mycoplasma infection.
Depressive symptoms
Symptoms were assessed in the third trimester (28–30 weeks gestation) using the BDI. The BDI is a 21-item scale that assesses depressive symptomatology.60 Each item is answered on a four-point scale, which indicates degree of severity. The BDI has been validated for use in pregnant and postpartum women and the sensitivity and specificity was 83% and 89%, respectively.61 Possible range of scores is 0 to 63, and the index score can be used as a continuous variable to assess depressive symptoms. Alternatively, a cutoff score can be used to distinguish mild depressive symptomatology (BDI score ≥10) or moderate to severe depressive symptomatology (BDI score ≥19). The sensitivity of the BDI is 100% for the 9/10 cutoff and 47.6% for the 18/19 cutoff.
Depression symptoms were assessed again 6–10 weeks postpartum with a different depression screening tool known as the 21-item Center for Epidemiologic Studies Depression (CES-D) Scale. Possible range of scores is 0 to 60, with the higher scores indicating the presence of more symptomatology.62 The CES-D was used as a dichotomous variable (CES-D ≥ 16) to represent risk for PPD (and is not diagnostic). The sensitivity of the CES-D to detect risk for PPD is only 60%, suggesting that the tool misses a significant number of depressed women in this time period. However, the specificity value of 92% suggests relatively high credibility.63
Vitamin D 25(OH)D Assay and 24, 25(OH)2D Assay
Maternal plasma samples were drawn in the second trimester (18–20 weeks gestation) in duplicate for quantitative measurements of vitamin D metabolites. These were centrifuged at 1,430 × g for 15 minutes, and 1.8 mL of serum was transferred to Nunc Cryotubes and stored at −80°C for future analysis. Approximately 100 μL of serum was mixed with 25(OH)D3–[2H6] and 24R,25-(OH)2D3–[2H6] isotopic internal standards dissolved in 5% bovine serum albumin (IsoSciences, Inc., King of Prussia, PA, USA). Total 25(OH)D3 and 24,25(OH)D3 were extracted away from DBP and other serum binding factors by protein precipitation with 250 μL methanol and cleared by centrifugation. Vitamin D metabolites were isolated from extracted supernatants by solid phase extraction chromatography (Strata C-18E 96-well SPE plates; Phenomenex, Inc., Torrance, CA, USA), and eluted with 1 mL ethyl acetate containing 0.1 mg/mL 4-Phenyl-1,2,4-triazole-3,5-dione (PTAD). PTAD-derivatized samples were dried under vacuum and redissolved with 100 μL of 50% ethanol.
Samples were then analyzed for vitamin D metabolites using reverse-phase liquid chromatography coupled to tandem mass spectrometry in multiple reaction monitoring mode (LC-MS/MS) [intra-assay coefficient of variation (CV) 1.1% and 3.5% for 25(OH)D3 and 24,25(OH)2D3, respectively]. Assays were calibrated using 25(OH)D3 and 24R,25-(OH)2D3 commercial standards (Cerilliant, Inc., Round Rock, TX, USA). Intact parathyroid hormone levels were measured using the Cobas electrochemiluminescense immunoassay on the Modular Analytics E170 automated analyzer (Roche Diagnostics, Indianapolis, IN, USA; inter-assay CV 2.5%). Additional method details are described elsewhere.52
Statistical analysis
Numerical variables were summarized by mean and standard deviation or median and range. Categorical variables were summarized by counts and percentages. Numerical variables were compared across groups by the independent samples t-test or the Wilcoxon rank sum test. Categorical variables were compared across groups by the Fisher's exact test. Stepwise multiple logistic regression models were used to assess the association between the potential predictor variables: VMR, BMI, maternal age, smoking, race (Hispanic/Latina vs. non-Hispanic/Latina), prenatal depression (BDI), and PPD (CES-D ≥ 16). A two-sided 0.05 significance level was used throughout. Statistical calculations were made using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Women in this study (N = 89) had complete prenatal and PPD data, and biomarker and demographic data, and were on average 27.80 years old (standard deviation = 5.87). The racial/ethnic distribution of participants was 42% Hispanic/Latina (n = 37), 29% black (n = 26), 27% white (n = 24), and 2% Asian (n = 2). None of the vitamin D metabolites we measured (including the VMR) significantly differed among the races, which was not an expected result. A contrast comparing total prenatal 25(OH)D3 in black women versus the average of Latina and white women may be suggestive (p = 0.111). A total of 30 women (34%) developed PPD and 59 women (66%) did not. Participant characteristics were stratified by having PPD for descriptive purposes (Table 1).
Table 1.
Demographic and Clinical Variables Stratified by Postpartum Depression (PPD Risk, CES-D ≥ 16) for Descriptive Purposes for N = 89 Women from the Behavior In Pregnancy Study
| Not at PPD risk |
PPD risk |
p | |
|---|---|---|---|
| N = 59 (66.3%) | N = 30 (33.7%) | ||
| Age (years) | 27.93 ± 6.00 | 27.53 ± 5.70 | 0.76 |
| Married | 33 (55.9) | 13 (43.3) | 0.27 |
| Education (years completed) | 13.5 ± 2.5 | 12.4 ± 2.7 | 0.086a |
| Race/ethnicity | 0.007 | ||
| White | 21 (35.6) | 3 (10) | |
| Black | 19 (32.2) | 7 (23.3) | |
| Hispanic/Latina | 18 (30.5) | 19 (63.3) | |
| Asian/Pacific Islander | 1 (1.7) | 1 (3.3) | |
| Parity | 0.95 ± 0.95 | 1.17 ± 1.05 | 0.33a |
| Multiparous | 38 (64.4) | 21 (70.0) | 0.64 |
| Height (inches) | 64. 3 ± 2.7 | 63.2 ± 2.8 | 0.089 |
| Prepregnancy weight (lbs.) | 143.07 ± 33.80 | 150.13 ± 43.86 | 0.40 |
| BMI | 24.37 ± 5.72 | 26.17 ± 6.08 | 0.17 |
| Fetal sex female | 32 (54.2) | 18 (60.0) | 0.66 |
| Smoker | 11 (18.0) | 7 (23.3) | 0.59 |
| Diabetic | 5 (8.5) | 0 (0) | 0.16 |
| Maternal infection | 15 (25.4) | 10 (33.3) | 0.46 |
| Birth weight (g) | 3397.5 ± 403.5 | 3401.5 ± 487.7 | 0.97 |
| Low birth weight (<2,500 g) | 2 (3.4) | 0 (0) | 0.55 |
| Prenatal 25(OH)D (ng/mL) | 20.63 ± 7.68 | 17.78 ± 7.00 | 0.093 |
| Prenatal 25(OH)D <20 ng/mL | 30 (50.8) | 17 (56.7) | 0.66 |
| Prenatal 24,25(OH)2D ng/mL | 2.19 ± 1.12 | 1.55 ± 0.73 | 0.002 |
| Prenatal PTH pg/mL | 22.46 ± 13.47 | 24.61 ± 11.80 | 0.46 |
| Prenatal BDIb | 12.4 ± 10.8 | 14.9 ± 11.8 | 0.22a |
| Prenatal BDI ≥10 | 27 (45.8) | 19 (63.3) | 0.18 |
| Postpartum CES-Dc | 8.7 ± 3.6 | 22.0 ± 6.7 | NA |
| Pregnancy VMR | 0.102 ± 0.023 | 0.087 ± 0.017 | 0.003 |
Data are N (%) or mean ± standard deviation, unless otherwise noted.
Comparison was tested with t-test or Fisher's exact test, unless otherwise noted. Statistically significant differences are in bold.
Wilcoxon rank sum test.
BDI mild depressive symptomatology (BDI score ≥10) or moderate to severe depressive symptomatology (BDI score ≥19).
CES-D Scale. A total of 16 or above is indicative of risk for clinical depression.
BDI, Beck Depression Inventory; CES-D, Center for Epidemiological Studies Depression; NA, not appropriate; PPD, postpartum depression; PTH, parathyroid hormone; VMR, Vitamin D Metabolite Ratio.
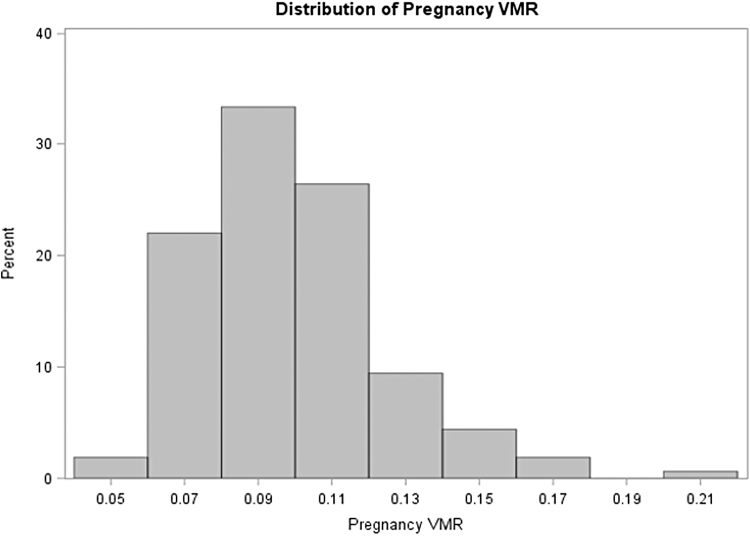
The only demographic or medical variable that significantly differed between the two groups was race, p = 0.007. Specifically, more Hispanic/Latina women had PPD (51.4%) compared to white women who had PPD (12.5%), p = 0.003. The only biomarkers that significantly differed between the two groups were prenatal 24,25(OH)2D ng/mL and the VMR, which ranged from 0.048 to 0.214 (Fig. 1). Women with PPD had lower VMR (0.087 ± 0.017) than women without PPD (0.102 ± 0.023), p = 0.003.
FIG. 1.
The Distribution of prenatal VMR, a new candidate biomarker for vitamin D status in the BIPS study (N = 89). Women with PPD had lower VMR (0.087 ± 0.017) than women without PPD (0.012 ± 0.023), p = 0.003. BIPS, Behavior In Pregnancy Study; PPD, postpartum depression; VMR, Vitamin D Metabolite Ratio.
After examining the association between demographic and medical variables with depressive symptoms, we determined whether these should be included in the model as potential confounders. The significant association between race and PPD led to the inclusion of race (Hispanic/Latina vs. non-Hispanic/Latina), in analyses. Theoretically derived confounding variables included BMI, maternal age, smoking, and BDI (risk for prenatal depression). Stepwise multiple logistic regression models were carried out with the following predictor variables: VMR, BMI, maternal age, smoking, race (Hispanic/Latina vs. non-Hispanic/Latina), and BDI (risk for prenatal depression). These predictor variables were included because they are either consistently associated with PPD in the literature (e.g., prenatal depression, high BMI) or because they were associated with PPD risk in this multivariable model (e.g., race and education).
Results showed that only lower VMR and Hispanic/Latina identification were significantly associated with higher risk for PPD (CES-D ≥ 16), OR = 1.43 (for a decrease of 0.01 on a scale of 0.048–0.214), 95% CI 1.10–1.86, p = 0.007, and OR = 3.83, 95% CI 1.44–10.92, p = 0.007, respectively. We created three separate models to determine whether lower vitamin D status predicted risk for PPD above and beyond these other important predictor variables.
Model 1: Logistic regression results from Model 1 showed that when Latina race, prenatal depression, and BMI were included, only Latina race was significantly associated with higher PPD (p = 0.004). Prenatal depression approached significance (p = 0.079) and BMI was not associated with PPD (p = 0.46).
Model 2: We removed BMI as it was not associated with PPD and we can only have three predictor variables in any model (N = 30 PPD cases total). Again, Latina race was significantly associated with PPD (p = 0.004) and prenatal depression approached significance (p = 0.073).
Model 3: We added the VMR and found that again Latina race was significantly associated with PPD (p = 0.004) and prenatal depression approached significance (p = 0.053), and VMR was also significantly associated with PPD (p = 0.006). Adding the VMR in Model 3 as another predictor variable improved the model fit [Delta (−2LogL) = 9.291, p = 0.023], and the C statistic was larger as well (0.785 vs. 0.709). The OR = 1.43 (for a decrease of 0.01 on a scale of 0.048 to 0.214), 95% CI 1.10–1.86, p = 0.007.
Finally, because the significance of the difference in 25(OH)D concentrations between darker skinned and white Americans is still a matter of investigation, we tested whether there are also differences in concentrations of 25(OH)D in this racially diverse sample. We found no evidence that lower levels of circulating 25(OH)D (continuous) or 25(OH)D <20 ng/mL were significantly associated with PPD risk; however, these lower levels were predominant in non-white women. In addition, lower VMR was significantly associated with risk for PPD and the VMR did not differ among races.
Discussion
In line with national rates of PPD in ethnic minorities (comprising 73% of our sample), our results showed that 34% of our sample were at risk for PPD and 66% were not. Results revealed that lower prenatal vitamin D levels, as measured by the VMR, predicted risk for PPD. The VMR represents a new, sensitive and robust multiplex LC-MS/MS assay for simultaneous measurement of 24,25(OH)2D, and 25(OH)D. Using the VMR will enhance future vitamin D research by using only optimal combination of analytes for the assessment of vitamin D sufficiency. Additional studies are needed to elucidate whether this relationship is causal and to explore and test mechanisms by which hypovitaminosis D predicts PPD.
Strengths, limitations, and future directions
The main strength of the BIPS was the prospective design. Women in this sample were studied in mid-pregnancy, at the time of their delivery, and 6–10 weeks postdelivery. An additional strength was the inclusion of important covariates such as maternal age, race/ethnicity, and medical variables. Limitations of this secondary data analysis include missing data on postpartum depressive symptoms because a large portion of the participants did not return for the final research visit. However, the percentage of women with elevated PPD scores in our sample is consistent with previous research, including racially diverse samples.64 In addition, the participants who were dropped from analyses due to lack of CES-D scores did not differ on age and 25(OH)D levels and had comparable prenatal BDI scores to those included in analyses. Therefore, underestimates of PPD symptoms due to attrition are less likely. Also, this study does not involve confirmed cases (diagnoses) of depression and future research should include diagnostic interviews. Finally, when symptom screening tools are used to identify PPD, the Edinburgh Postnatal Depression Scale is recommended for research and clinical use. The BDI and CES-D, however, demonstrate good to excellent internal consistency with postpartum samples.65
Future work will focus on mechanisms of action. For example, vitamin D inhibits cytokine cross talk, thus repressing exaggerated inflammatory responses. Adams et al.36 significantly added to this field of research by showing that human immune cells respond to bacterial cell walls by building both VDR proteins and the enzyme that converts circulating 25(OH)D into the biologically active form.36 In two recent RCTs with nonpregnant samples, vitamin D3 supplementation reduced anxiety and depressive symptoms,66,67 and lowered inflammation compared to placebo,67 suggesting a putative role for vitamin D deficiency in inflammatory induced depression.
Vitamin D deficiency during pregnancy and its role in depression and other perinatal adverse outcomes have only recently been given attention. Vitamin D regulates placental development and function, and promotes tolerance of the fetus, and deficiency is associated with preeclampsia and reduced infant birth size, among other adverse birth outcomes.34,36,38,68–70 Indeed, a recent study from our team reported that prenatal vitamin D deficiency was associated with developing an adverse perinatal outcome compared to those vitamin D sufficient. Furthermore, this higher rate of adverse perinatal outcome was discovered when women had both prenatal vitamin D deficiency and elevated depressive symptoms.71 Therefore, vitamin D supplementation, which is a safe and cost-effective intervention during pregnancy,72 may benefit both the mother's health and that of the developing fetus.73–78
In a randomized control trial, vitamin D supplementation during pregnancy lowered the inflammation marker tumor necrosis factor-alpha and the markers of endothelial activation. The supplementation also enhanced the percentage of CD4+ and IL-10+T cells in peripheral blood, reducing the excess inflammation and improving the pregnancy outcome.79 It has also been shown that metabolism of 25(OH)D to 1,25(OH)2D promotes antibacterial and anti-inflammatory responses in both the maternal decidua80 and the fetal trophoblast.81,82 Immunomodulatory actions of vitamin D are expected to be compromised when maternal 25OHD3 status is low, with potentially detrimental inflammatory conditions.
Recently, one of the largest (N = 1,024), most racially diverse trials of vitamin D supplementation found that pregnant women with 25(OH)D ≥40 ng/mL had a 62% lower risk of PTB compared to those with <20 ng/mL (p < 0.0001). This lowered risk remained even after adjusting for socioeconomic variables (OR = 0.41, p = 0.002). The authors reported a significantly lower PTB rate (60%) for those who reached ≥40 ng/mL versus those who did not (78%), particularly among women of color.83 These data imply that vitamin D plays a critical role in controlling fetal-placental immune responses during pregnancy. Therefore, improving the vitamin D status of women of color, who have particularly low vitamin D concentrations, might decrease racial disparities in PTB rates and could decrease PPD rates as well.
Randomized controlled trials of prenatal vitamin D supplementation to reduce PPD are therefore warranted, and to our knowledge, only two such trials have been conducted. Vaziri et al. showed that supplementing with 2,000 IU vitamin D3 daily during late pregnancy effectively decreased perinatal depressive symptoms.78 Rouhi et al. reported that taking 1,000 IU vitamin D3 daily for 6 months significantly reduced depression scores and fatigue in 80 primiparous women.84 Routine measurement of prenatal vitamin D levels would therefore allow for early maternal mental health intervention, which could benefit the mother and her entire family.85 Furthermore, best practice would include prenatal testing for blood levels of all the metabolites needed for measurement of the VMR both before and after supplementation to ensure that a woman has sufficient levels, regardless of the season.
Summary
Vitamin D insufficiency as measured by the VMR is associated with higher risk for PPD. Women with darker skin frequently have lower total 25(OH)D without indication of vitamin D deficiency, suggesting that total serum 25(OH)D may be misleading by incorrectly reflecting vitamin D status in different racial groups. A new candidate biomarker for vitamin D status is the ratio of serum 24,25(OH)2D to 25(OH)D (VMR), which is robust and not sensitive to racial differences. Routine prenatal screening for vitamin D metabolites, particularly in Hispanic/Latina women, may identify women at risk for PPD.
Acknowledgments
We thank the participants in this study for contributing to this research and increasing our knowledge about the experiences of pregnant women. We acknowledge the support of the research team members, Rae Buttle, BA, and Morgan McKay, MSW at Cedars-Sinai.
We thank Cedars-Sinai Obstetrics and Gynecology Department and Los Angeles Helping Hand Miriam Jacobs Chair Maternal Fetal Medicine.
Authors' Contributions
E.E.A. developed the research question for secondary analyses and wrote the first draft of this article. A.H.B. and S.A.K. provided VMR expertise and assayed the samples. J.M. analyzed the data. C.A., C.J.H., S.J., R.L., A.H.B., S.A.K., and J.M. assisted in writing. All authors contributed to this study design and interpretation of data, and approved the final article. The authors have no financial gain related to the outcome of this research, and there are no potential conflicts of interest.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Statistical support was funded by the National Center for Advancing Translational Sciences (NCATS) Grant UL1TR001881. The parent study was supported by funding by an NIH award No. RO1 HD-11753 from the National Institutes of Child Health and Human Development to C.J.H. and C.D.C and the Miriam Jacobs Chair in Maternal Fetal Medicine (C.J.H.).
References
- 1. O'Hara MW, McCabe JE. Postpartum depression: Current status and future directions. Annu Rev Clin Psychol 2013;9:379–407 [DOI] [PubMed] [Google Scholar]
- 2. Gjerdingen D, McGovern P, Attanasio L, Johnson PJ, Kozhimannil KB. Maternal depressive symptoms, employment, and social support. J Am Board Fam Med 2014;27:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norhayati MN, Hazlina NH, Asrenee AR, Emilin WM. Magnitude and risk factors for postpartum symptoms: A literature review. J Affect Disord 2015;175:34–52 [DOI] [PubMed] [Google Scholar]
- 4. Ceballos M, Wallace G, Goodwin G. Postpartum depression among African-American and Latina mothers living in small cities, towns, and rural communities. J Racial Ethn Health Disparities 2017;4:916–927 [DOI] [PubMed] [Google Scholar]
- 5. Orr ST, Blazer DG, James SA. Racial disparities in elevated prenatal depressive symptoms among black and white women in eastern North Carolina. Ann Epidemiol 2006;16:463–468 [DOI] [PubMed] [Google Scholar]
- 6. Ramos-Marcuse F, Oberlander SE, Papas MA, McNary SW, Hurley KM, Black MM. Stability of maternal depressive symptoms among urban, low-income, African American adolescent mothers. J Affect Disord 2010;122:68–75 [DOI] [PubMed] [Google Scholar]
- 7. Lucero NB, Beckstrand RL, Callister LC, Sanchez Birkhead AC. Prevalence of postpartum depression among Hispanic immigrant women. J Am Acad Nurse Pract 2012;24:726–734 [DOI] [PubMed] [Google Scholar]
- 8. Segre LS, O'Hara MW, Losch ME. Race/ethnicity and perinatal depressed mood. J Reprod Infant Psychol 2006;24:99–106 [Google Scholar]
- 9. Howell EA, Mora P, Leventhal H. Correlates of early postpartum depressive symptoms. Matern Child Health J 2006;10:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersson L, Sundstrom-Poromaa I, Bixo M, Wulff M, Bondestam K, Åström M. Point prevalence of psychiatric disorders during the second trimester of pregnancy: A population-based study. Am J Obstet Gynecol 2003;189:148–154 [DOI] [PubMed] [Google Scholar]
- 11. Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: Systematic review. Obstet Gynecol 2004;103:698–709 [DOI] [PubMed] [Google Scholar]
- 12. Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol 2007;110:1102–1112 [DOI] [PubMed] [Google Scholar]
- 13. McDonald SW, Lyon AW, Benzies KM, et al. . The All Our Babies pregnancy cohort: Design, methods, and participant characteristics. BMC Pregnancy Childbirth 2013;13(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: Relationship to poor health behaviors. Am J Obstet Gynecol 1989;160(5 Pt 1):1107–1111 [DOI] [PubMed] [Google Scholar]
- 15. Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry 2002;63(Suppl 7):9–15 [PubMed] [Google Scholar]
- 16. Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: A review. Arch Womens Mental Health 2012;15:1–14 [DOI] [PubMed] [Google Scholar]
- 17. Ding XX, Wu YL, Xu SJ, et al. . Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J Affect Disord 2014;159:103–110 [DOI] [PubMed] [Google Scholar]
- 18. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010;67:1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szegda K, Markenson G, Bertone-Johnson ER, Chasan-Taber L. Depression during pregnancy: A risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med 2014;27:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Accortt EE, Cheadle AC, Dunkel Schetter C. Prenatal depression and adverse birth outcomes: An updated systematic review. Matern Child Health J 2015;19:1306–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amini S, Jafarirad S, Amani R. Postpartum depression and vitamin D: A systematic review. Crit Rev Food Sci Nutr 2019;59:1514–1520 [DOI] [PubMed] [Google Scholar]
- 22. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br J Psychiatry 2013;202:100–107 [DOI] [PubMed] [Google Scholar]
- 23. Gur EB, Gokduman A, Turan GA, et al. . Mid-pregnancy vitamin D levels and postpartum depression. Eur J Obstet Gynecol Reprod Biol 2014;179:110–116 [DOI] [PubMed] [Google Scholar]
- 24. Nielsen NO, Strom M, Boyd HA, et al. . Vitamin D status during pregnancy and the risk of subsequent postpartum depression: A case-control study. PLOS One 2013;8:e80686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson M, Whitehouse AJ, Newnham JP, et al. . Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch Womens Ment Health 2014;17:213–219 [DOI] [PubMed] [Google Scholar]
- 26. Brandenbarg J, Vrijkotte TG, Goedhart G, van Eijsden M. Maternal early-pregnancy vitamin D status is associated with maternal depressive symptoms in the Amsterdam Born Children and Their Development cohort. Psychosom Med 2012;74:751–757 [DOI] [PubMed] [Google Scholar]
- 27. Cassidy-Bushrow AE, Peters RM, Johnson DA, Li J, Rao DS. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J Womens Health 2012;21:1189–1195 [DOI] [PubMed] [Google Scholar]
- 28. Huang JY, Arnold D, Qiu CF, Miller RS, Williams MA, Enquobahrie DA. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J Womens Health 2014;23:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamb A, Hobel C, Pepkowitz S, et al. . 763: Vitamin D deficiency and depressive symptoms in the perinatal period: A prospective study. Am J Obstet Gynecol 2014;212:S371. [DOI] [PubMed] [Google Scholar]
- 30. Williams JA, Romero VC, Clinton CM, et al. . Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregn Childbirth 2016;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu CW, Liu JT, Tu WJ, Yang JQ, Cao Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG 2015;122:1688–1694 [DOI] [PubMed] [Google Scholar]
- 32. Murphy PK, Mueller M, Hulsey TC, Ebeling MD, Wagner CL. An exploratory study of postpartum depression and vitamin d. Journal of the Am Psychiatric Nurs Assoc 2010;16:170–177 [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Liu N, Sun W, Chen D, Zhao J, Zhang W. Association between vitamin D deficiency and antepartum and postpartum depression: A systematic review and meta-analysis of longitudinal studies. Arch Gynecol Obstet 2018;298:1045–1059 [DOI] [PubMed] [Google Scholar]
- 34. Liu PT, Stenger S, Li H, et al. . Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 35. Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–322 [DOI] [PubMed] [Google Scholar]
- 36. Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N York Acad Sci 2007;1117:94–105 [DOI] [PubMed] [Google Scholar]
- 37. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alfaham M, Woodhead S, Pask G, Davies D. Vitamin D deficiency: A concern in pregnant Asian women. Br J Nutr 1995;73:881–887 [DOI] [PubMed] [Google Scholar]
- 39. Grover SR, Morley R. Vitamin D deficiency in veiled or dark-skinned pregnant women. Med J Aust 2001;175:251–252 [DOI] [PubMed] [Google Scholar]
- 40. Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 2005;81:1060–1064 [DOI] [PubMed] [Google Scholar]
- 41. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporosis Int 2011;22:1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract 2012;18:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137:447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Meer IM, Karamali NS, Boeke AJ, et al. . High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 2006;84:350–353; quiz 468-469. [DOI] [PubMed] [Google Scholar]
- 46. Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: A population-based study of younger and older adults. Am J Med 2004;116:634–639 [DOI] [PubMed] [Google Scholar]
- 47. Cauley JA, Danielson ME, Boudreau R, et al. . Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: The Women's Health Initiative (WHI). J Bone Miner Res 2011;26:2378–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cauley JA, Lui LY, Ensrud KE, et al. . Bone mineral density and the risk of incident nonspinal fractures in black and white women. J Am Med Assoc 2005;293:2102–2108 [DOI] [PubMed] [Google Scholar]
- 49. Hannan MT, Litman HJ, Araujo AB, et al. . Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 2008;93:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aloia J, Mikhail M, Dhaliwal R, et al. . Free 25(OH)D and the Vitamin D Paradox in African Americans. J Clin Endocrinol Metab 2015;100:3356–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Powe CE, Evans MK, Wenger J, et al. . Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berg AH, Powe CE, Evans MK, et al. . 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin Chem 2015;61:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bosworth CR, Levin G, Robinson-Cohen C, et al. . The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 2012;82:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wagner D, Hanwell HE, Schnabl K, et al. . The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol 2011;126:72–77 [DOI] [PubMed] [Google Scholar]
- 55. de Boer IH, Sachs MC, Chonchol M, et al. . Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: A participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis 2014;64:187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 2014;55:13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pike JW, Meyer MB. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch Biochem Biophys 2012;523:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hobel C, Dunkel-Schetter C, Roesch S. Maternal stress as a signal to the fetus. Prenatal Neonatal Med 1998;3:116–120 [Google Scholar]
- 59. Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks' gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol 1999;180(1 Pt 3):S257–S263 [DOI] [PubMed] [Google Scholar]
- 60. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 61. Holcomb WL Jr., Stone LS, Lustman PJ, Gavard JA, Mostello DJ.. Screening for depression in pregnancy: Characteristics of the Beck Depression Inventory. Obstet Gynecol 1996;88:1021–1025 [DOI] [PubMed] [Google Scholar]
- 62. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure 1977;1:15 [Google Scholar]
- 63. Campbell SB, Cohn JF. Prevalence and correlates of postpartum depression in first-time mothers. J Abnorm Psychol 1991;100:594–599 [DOI] [PubMed] [Google Scholar]
- 64. Dolbier C, Rush T, Sahadeo L, Shaffer M, Thorp J. Relationships of race and socioeconomic status to postpartum depressive symptoms in rural African American and Non-Hispanic White women. Matern Child Health J 2013;17:1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boyd RC, Le HN, Somberg R. Review of screening instruments for postpartum depression. Arch Womens Mental Health 2005;8:141–153 [DOI] [PubMed] [Google Scholar]
- 66. Eid A, Khoja S, AlGhamdi S, et al. . Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis 2019;34:1781–1786 [DOI] [PubMed] [Google Scholar]
- 67. Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in Vitamin D deficient Type 2 diabetic women with anxiety: A randomized clinical trial. Int J Prev Med 2019;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 2007;92:3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 2006;91:906–912 [DOI] [PubMed] [Google Scholar]
- 70. Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest 1987;79:1659–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Accortt EE, Lamb A, Mirocha J, Hobel CJ. Vitamin D deficiency and depressive symptoms in pregnancy are associated with adverse perinatal outcomes. J Behav Med 2018;41:680–689 [DOI] [PubMed] [Google Scholar]
- 72. Hollis BW, Wagner CL. Vitamin D requirements and supplementation during pregnancy. Curr Opin Endocrinol Diabetes Obes 2011;18:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J Nutr 2013;143:1432–1438 [DOI] [PubMed] [Google Scholar]
- 74. De-Regil LM, Palacios C, Lombardo LK, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2016;1:CD008873. [DOI] [PubMed] [Google Scholar]
- 75. Kalra P, Das V, Agarwal A, et al. . Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr 2012;108:1052–1058 [DOI] [PubMed] [Google Scholar]
- 76. Perez-Lopez FR, Pasupuleti V, Mezones-Holguin E, et al. . Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2015;103:1278–1288.e4. [DOI] [PubMed] [Google Scholar]
- 77. Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ 2017;359:j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, Sharif F, Jafari P. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregn Childbirth 2016;16:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J Nutr 2016;146:2388–2397 [DOI] [PubMed] [Google Scholar]
- 80. Delvin EE, Gagnon L, Arabian A, Gibb W. Influence of calcitriol on prolactin and prostaglandin production by human78 decidua. Mol Cell Endocrinol 1990;71:177–183 [DOI] [PubMed] [Google Scholar]
- 81. Diaz L, Noyola-Martinez N, Barrera D, et al. . Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. Am J Reprod Immunol 2009;81:17–24 [DOI] [PubMed] [Google Scholar]
- 82. Ross R, Florer J, Halbert K, McIntyre L. Characterization of 1,25-dihydroxyvitamin D3 receptors and in vivo targeting of [3H]-1,25(OH)2D3 in the sheep placenta. Placenta 1989;10:553–567 [DOI] [PubMed] [Google Scholar]
- 83. McDonnell SL, Baggerly KA, Baggerly CA, et al. . Maternal 25(OH)D concentrations >/ = 40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS One 2017;12:e0180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rouhi MRN, Mohamadpour S, Tajrishi HP. Vitamin D reduces postpartum depression and fatigue among Iranian women. Br J Midwif 2018;26:7 [Google Scholar]
- 85. Hobel CJ. Vitamin D supplementation should be routine in pregnancy: FOR: Recent research supports routine vitamin D supplementation in pregnancy. BJOG 2015;122:1021. [DOI] [PubMed] [Google Scholar]