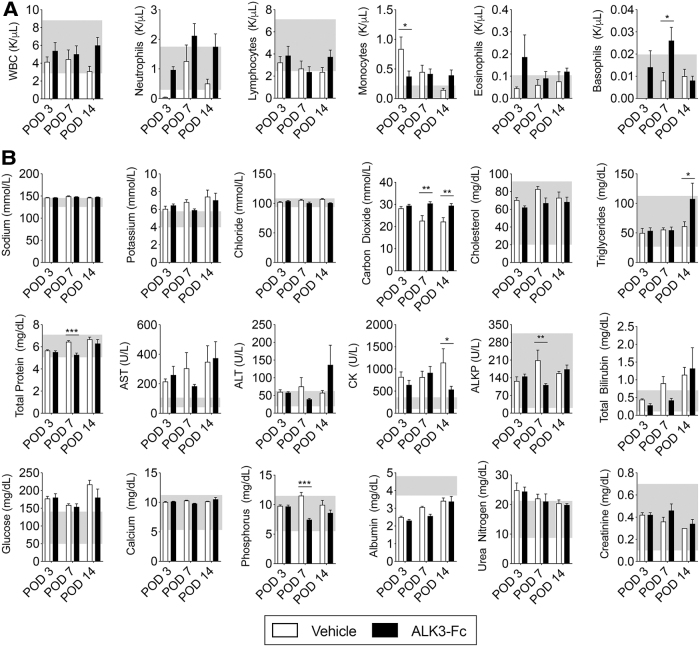
FIG. 3.
No systemic side effects following treatment with ALK3-Fc. Following blast-related polytraumatic extremity injury and MRSA infection, rats were treated with vehicle (PBS, n = 3) or ALK3-Fc (3 mg/kg, n = 3) twice a week with intraperitoneal injections and whole blood was analyzed on POD3, POD7, and POD14. Blood was collected from the tail vein and immediately subjected to analysis. (A) Complete blood count and (B) comprehensive metabolic profile were evaluated. Mean ± SEM. *P < 0.05, **P < 0.01, comparing vehicle and ALK3-Fc treatment. Gray box indicates normal references values. WBC, white blood count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactic acid dehydrogenase; CK, creatine kinase, ALP, alkaline phosphatase.