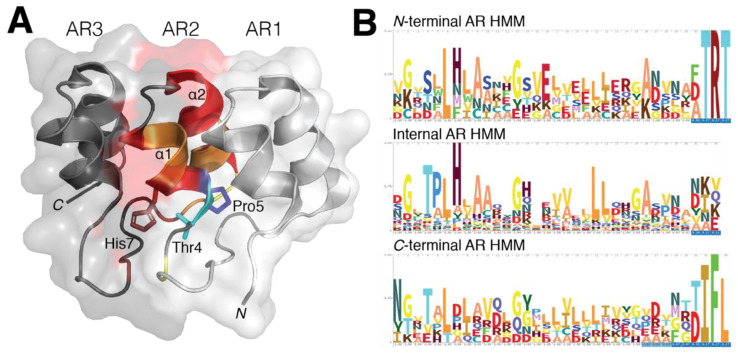
Figure 1.
Structural conservation within ankyrin repeat domains. (A) Residues Thr4, Pro5 and His7 play a critical role in providing both the 90° and L-shape formation through hydrogen bonding. The elongated solenoid shape of an AR domain is predominantly dictated by these three residues while hydrophobic interactions in the core of the AR are required to stabilize the domain’s 3D fold. (B) The hidden Markov model (HMM) profile of the AR-containing proteins for N-terminal, internal and C-terminal repeats were analyzed to highlight the occurrence of residues in identified AR domain families. The classic G-X-TPLHLA motif was readily identified to have a strong probability of occurring in these AR-containing proteins, whereas in both N- and C-terminal repeats were observed to contain only portions of this motif. While conservation within AR domains is more prevalent within the internal and C-terminal repeats, the N-terminal AR still retains similar AR domain characteristics.