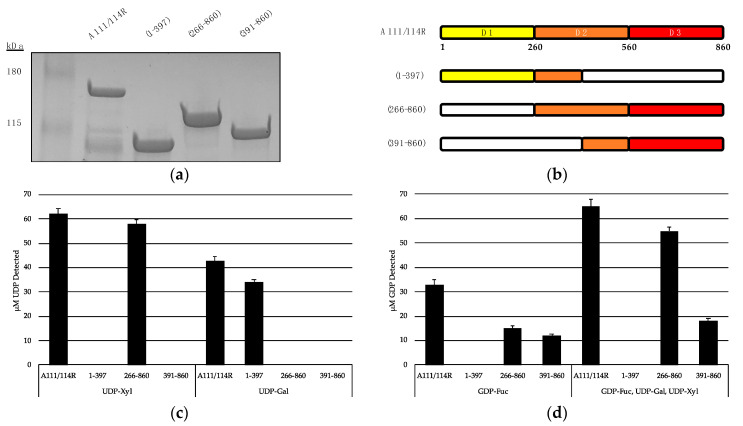
Figure 6.
Hydrolysis of UDP- and GDP-sugars by recombinant A111/114R and truncated constructs. (a) SDS-PAGE analysis of the expressed proteins: full length recombinant MBP-A111/114R (144 kDa), and truncated variants MBP-A111/114R (1–397) (87 kDa), MBP-A111/114R (266–860) (110 kDa), and MBP-A111/114R (391–860) (96 kDa) were eluted from an amylose column and resolved by SDS/PAGE with Coomassie blue staining. BenchMarkTM pre-stained protein ladder and recombinant proteins were separated on a 4–20% tris-glycine gel. (b) Cartoon renderings of the full-length A111/114R protein and truncated versions are color coordinated by three putative domains (D1, D2, D3), each corresponding to a different nucleotide sugar donor. White regions denote omitted sections of A111/114R. Shortened constructs are defined by their residues in the left column. (c and d) Representative data from hydrolysis assays shown as a ratio of the UDP (c) or GDP (d) measured from reactions containing the indicated GTs relative to the negative controls where no enzyme was added. The release of UDP and GDP was detected by the UDP-GloTM assay and GDP-GloTM assay, respectively. GDP-hydrolysis from GDP-Fuc was significantly elevated in the presence of UDP-Gal and UDP-Xyl with A111/114R (266–860) and A111/114R (391–860). Data are representative from three replicates, and error bars represent standard deviation.