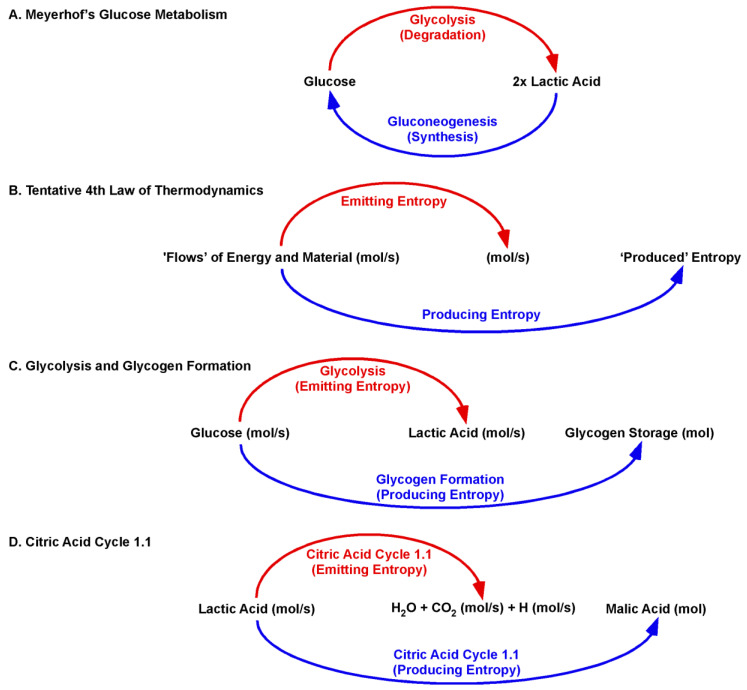
Figure 4.
From Chemistry to Biology in metabolism. (A) Glucose metabolism is illustrated as a chemical reaction. Meyerhof’s interpretation of the metabolism of glycolysis is based on strict stoichiometry. One molecule of glucose is converted to two molecules of lactic acid. The degradation reaction (glycolysis) and the reverse synthetic reaction (gluconeogenesis) are in equilibrium. (B) The tentative Fourth Law of Thermodynamics asserts that a flow of energy and material (emitting entropy) is sufficient to produce entropy. (C) By transferring the tentative Fourth Law of Thermodynamics (a law of nature) to Biology, glycolysis (emitting entropy) is sufficient to produce entropy (glycogen). The different physical quantities are key here: By setting glucose transporters as the first enzyme and proton-linked MCT4 as the final enzyme, glycolysis is a flow of energy and material (mol/s), whereas glycogen (mol) is a product of this flow. (D) The Citric Acid Cycle 1.1 makes the dynamics clear. If lactic acid is not “burned,” the import of lactic acid into the cycle is blocked.