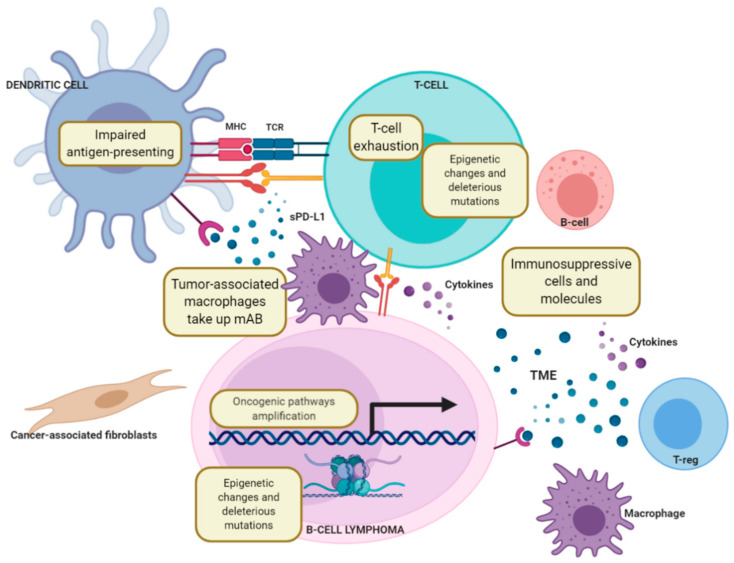
Figure 3.
Mechanisms of resistance to immune checkpoint blockade. The deregulation of MHC class I components such as the loss of β2 microglobulin (B2M) and the loss of human leukocyte antigen (HLA) heterozygosity as well as defects in IFN signalling pathways may impair antigen recognition by antitumor CD8+ T-cells. Amplification of oncogenic signalling pathways such as PI3K/AKT/mTOR, Wnt/β-catenin, and MAPK increases the production of immunosuppressive cytokines, trigger T-cell exclusion from TME and may also result in resistance to immune checkpoint blockade. Epigenetic (histone acetylation or DNA methylation) and genetic (deleterious mutations) alterations are crucial triggers of gene expression disorders related to sustained T-cell exhaustion that could eventually cause the failure of immune checkpoint therapy. Moreover, myeloid-derived suppressor cells (MDSCs), Tregs, tumour-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs) are major immunosuppressive cell types within the TME that may contribute to resistance to immune checkpoint blockade. Immunosuppressive molecules such as TGF-β and IFN-γ, secreted by tumour cells, myeloid cells and macrophages in the TME, may also suppress the functions of effector T-cells, rendering immune checkpoint blockade ineffective.