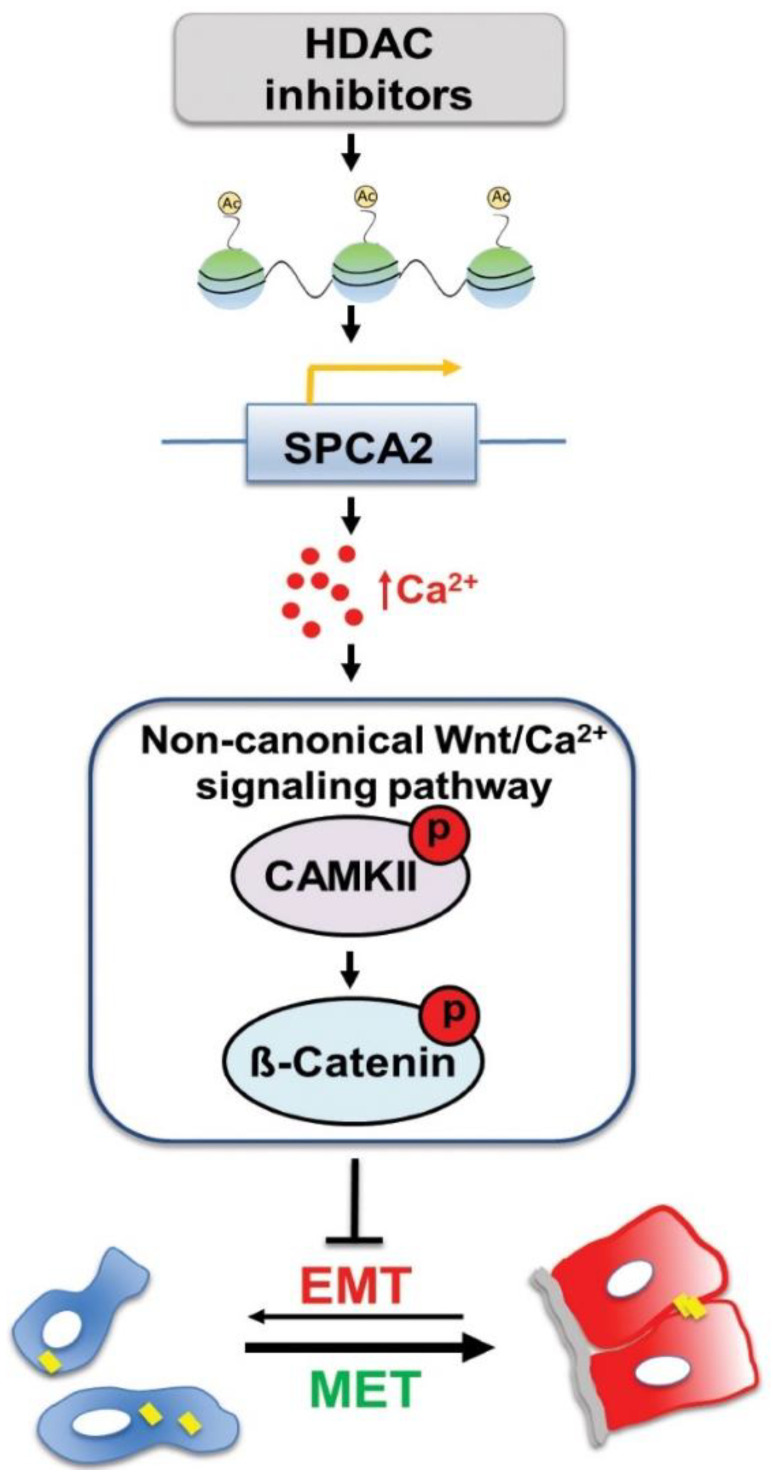
Figure 7.
Epigenetic modulation of SPCA2 in TNBC reverses EMT through Ca2+/Wnt signaling. Breast cancer cells undergo reversible transitions between mesenchymal state, characterized by low cytosolic Ca2+ (blue) and detached junctional protein E-cadherin (yellow bars), and epithelial state, characterized by higher resting Ca2+ (red), attachment to the basement membrane (gray), and cell-cell junctions (yellow). Previous work showed that ectopic expression of SPCA2 in TNBC cells increases cytosolic Ca2+ and E-cadherin expression to promote epithelial phenotypes. In this study, we show that HDAC inhibitors (vorinostat and romidepsin) increase histone acetylation leading to increased SPCA2 transcription, which in turn elevates resting Ca2+ by activation of store-independent Ca2+ entry. Ca2+ activates non-canonical Wnt signaling by phosphorylating CAMKII and β-catenin, turning Wnt OFF. This blocks EMT and promotes MET.