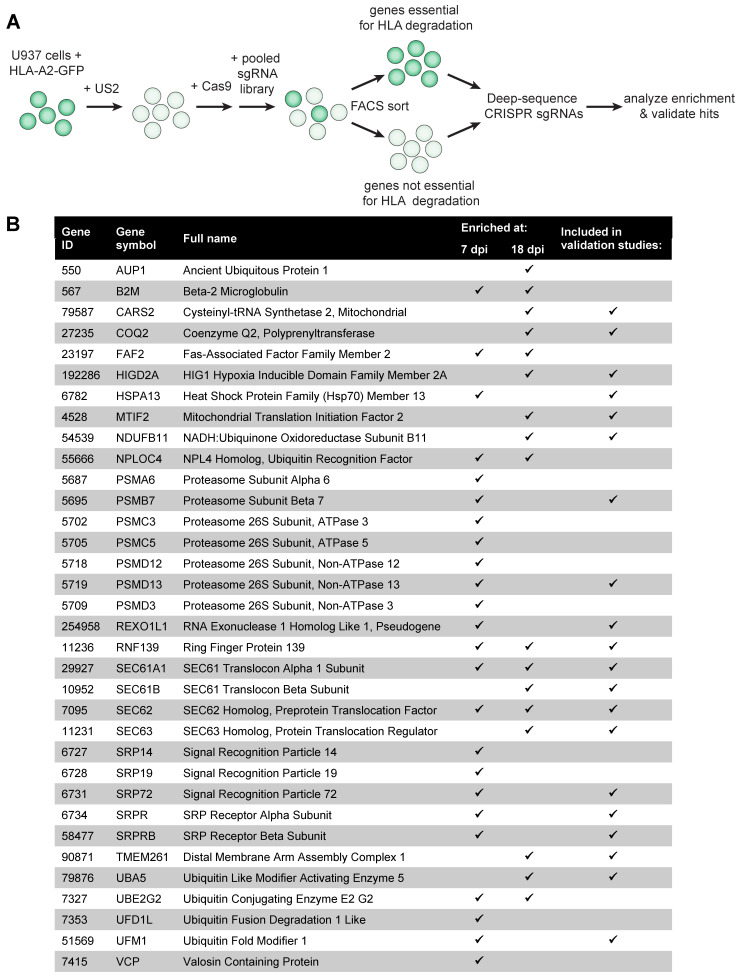
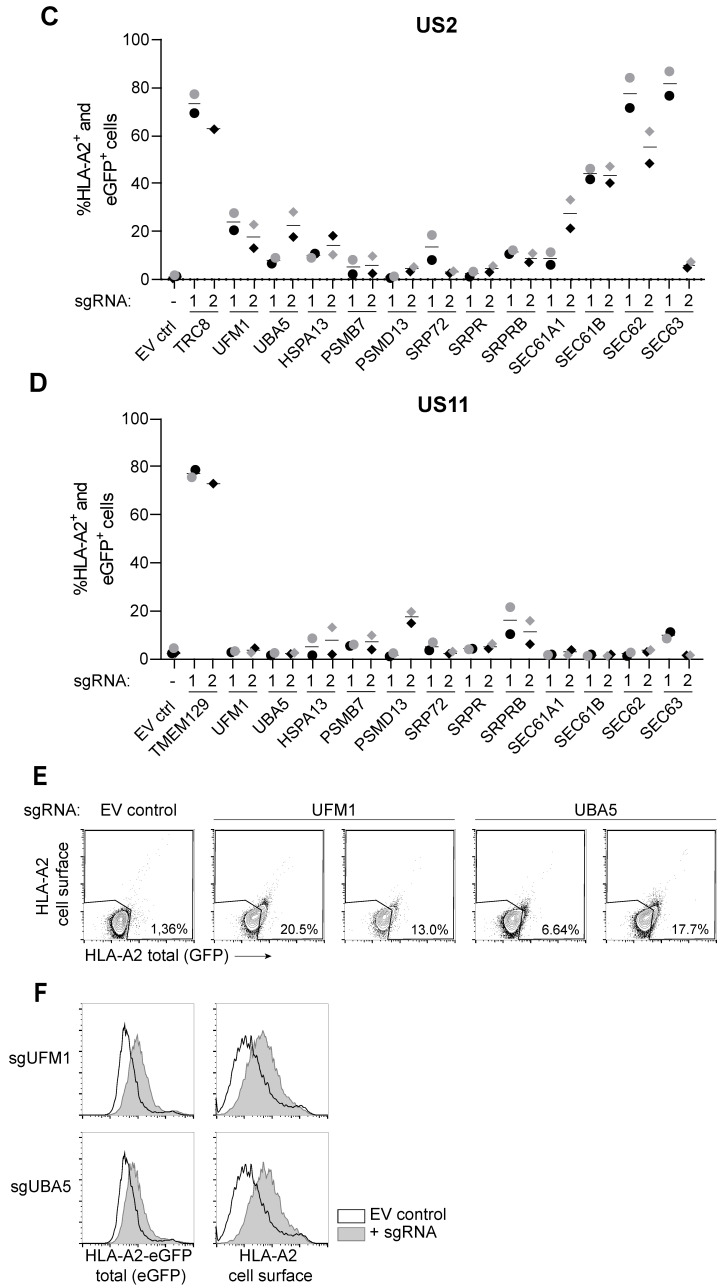
Figure 1.
A genome-wide CRISPR/Cas9 library screen identifies the UFM1 pathway to affect HCMV US2-mediated degradation of HLA class I. (A) Schematic overview of the genome-wide CRISPR/Cas9 library screen setup. (B) Overview of hits identified in the library screen and genes selected for validation studies. Flow cytometric analysis for the library screen was performed at 7- and 18 days post-lentiviral transduction with the sgRNA library. The checkmarks indicate at which timepoint the respective genes were identified. (C) Validation of the screen at 14 days post-transduction of the sgRNAs. HLA-A2-eGFP U937 cells co-expressing US2 and SpCas9 were transduced with single sgRNAs targeting the presented genes. For each gene, the two most enriched sgRNAs from the screen were validated. The ubiquitin E3 ligase TRC8 was included as positive control, and an empty vector (EV ctrl) was included as negative control. Rescue of HLA-A2-eGFP (as measured by assessing eGFP levels and cell surface staining with an HLA-A2-specific antibody) was measured by flow cytometry. Validation was performed twice at 7, 11, 14, 18, and 21/28 days post-infection. The other timepoints are shown in Figure S1. Circles (sgRNA1) and diamonds (sgRNA2) in black or grey represent two independent experiments. (D) Same as in (C), although US11-expressing U937 cells were tested, instead of US2-expressing cells. HLA-A2 rescue observed in the polyclonal knockout cells are specific to US2 for the majority of genes. TMEM129 was taken along as a positive control for these US11-expressing cells, as this ubiquitin ligase is essential for US11 function. (E) Flow cytometry dot plots of UBA5- and UFM1 sgRNA-targeted cells from the cells shown in Figure 1C. (F) UFM1- and UBA5-targeting sgRNAs were introduced in a different cell type (U373 cells), and rescue of HLA-A2-eGFP was measured by flow cytometry.