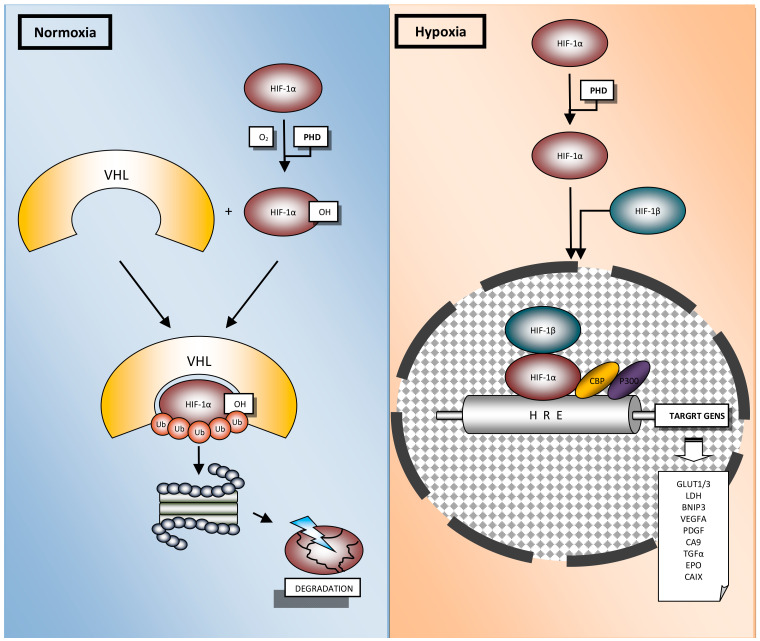
Figure 2.
Regulation of HIF-1α in physiological oxygen concentration (normoxia) and hypoxia. In normoxia, HIF-1α is rapidly degraded via ubiquitin-mediated process. The hydroxylation of HIF-1α by prolyl hydroxylase domain proteins (PHD), with use of oxygen, leads to interaction with Von Hippel–Lindau (VHL), addition of ubiquitin (Ub) and HIF-1α proteasomal degradation. Under oxygen deprivation, HIF-1α is not targeted for degradation. Hypoxia leads to HIF-1α accumulation in the cytoplasm, whence it is translocated to the nucleus where it binds with HIF-1β. α and β subunits form the heterodimer HIF-1, which joins to the hypoxia response element (HRE) and recruits co-activators (CBP, p300) at the HRE to induce the transcriptional activity of target genes.