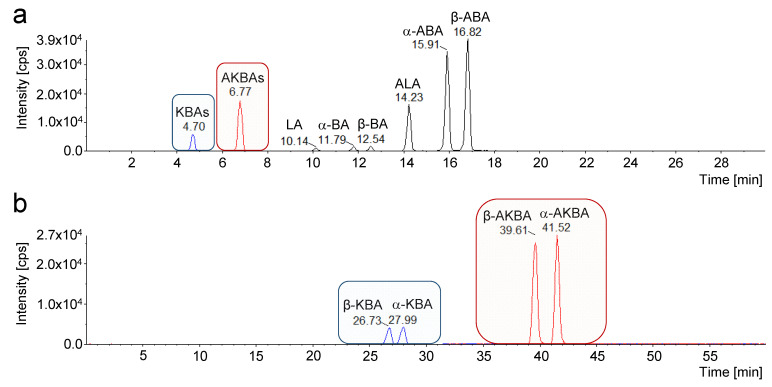
Figure 5.
Chromatographic separation of 11-keto-boswellic acids with different stationary phases. (a) Multiple reaction monitoring (MRM) chromatogram using a C18 stationary phase. The constitutional isomers lupeolic acid (LA), α-boswellic acid (α-BA), and β-boswellic acid (β-BA), as well as acetyl-lupeolic acid (ALA), acetyl-α-boswellic acid (α-ABA), and acetyl-β-boswellic acid (β-ABA), could be separated sufficiently. However, it is not possible to separate the constitutional isomers of 11-keto-boswellic acid (KBAs) and acetyl-11-keto-bowellic acid (AKBAs) under these conditions [5]. (b) MRM chromatogram using a pentafluorophenyl (PFP) stationary phase. The utilization of a PFP stationary phase and optimization of the chromatographic parameters by DoE enabled a successful separation and selective quantification of all KBAs and AKBAs isomers, namely 11-keto-α-boswellic acid (α-KBA), 11-keto-β-boswellic acid (β-KBA), acetyl-11-keto-α-boswellic acid (α-AKBA), and acetyl-11-keto-β-boswellic acid (β-AKBA).