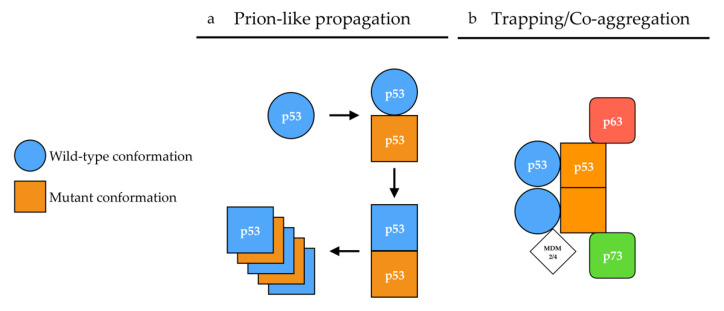
Figure 2.
Proposed mechanisms of p53 aggregation. (a) According to the prion-like hypothesis, mutant p53 (orange) in a [p53MUT] conformation (square) induces a conformational shift of wild-type p53 (blue) from a [p53WT] (circle) conformation toward a [p53MUT] conformation in a prion-like manner. In a [p53MUT] conformation, wild-type and mutant p53 form amyloid fibers. (b) According to the co-aggregation/trapping hypothesis, mutant p53 hetero-tetramerizes with wild-type p53. The unfolded core of mutant p53 allows new interactions with p63/p73 isoforms and MDM2/4 in a trapping/co-aggregation mechanism.