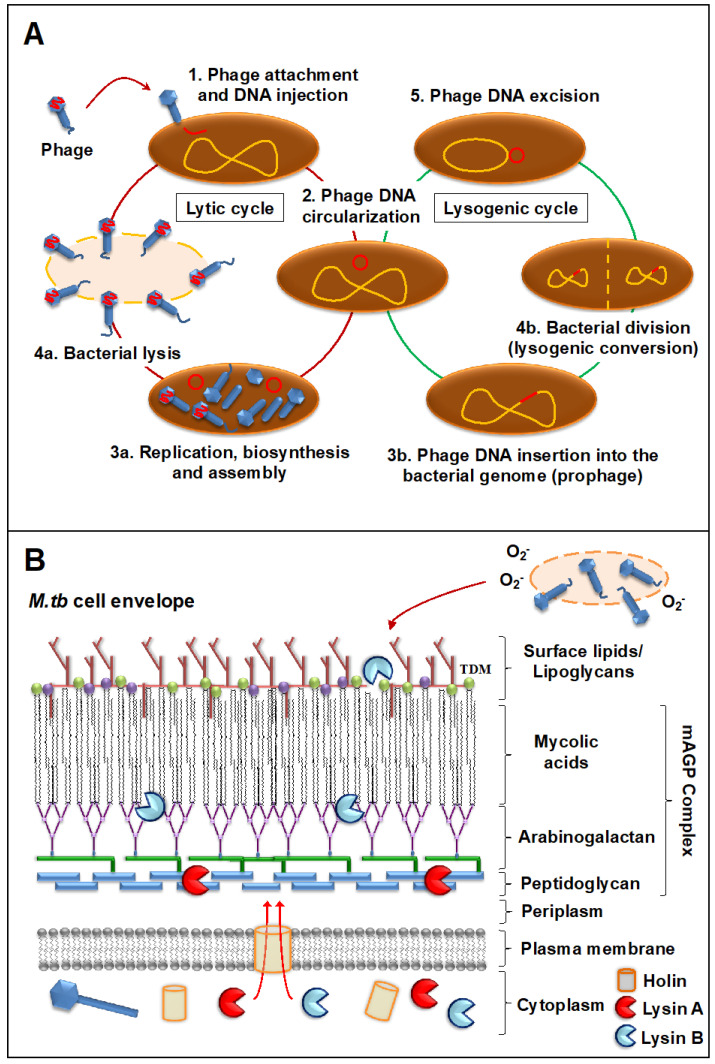
Figure 1.
Infection of M. tuberculosis by mycobacteriophages. (A) Steps during mycobacteriophage infection: (1) The phage attaches to M. tuberculosis through specific receptors and injects its DNA (red); (2) Phage DNA circularizes inside the M. tuberculosis bacillus; then, specific environmental cues will determine if the phage undergoes a lytic or a lysogenic cycle. If the lytic cycle is induced: (3a) New phage DNA and viral proteins are synthesized and assembled into new viral particles and; (4a) Viral particles will be released after the lysis of the M. tuberculosis bacillus. If the lysogenic cycle is induced: (3b) The phage genome is integrated into the M. tuberculosis chromosome, becoming a prophage; (4b) The prophage will replicate along the M. tuberculosis genome and will be transmitted to the progeny that will acquire new properties encoded in the prophage (lysogenic conversion); (6) Under certain triggers, the prophage DNA will be excisioned from the bacterial chromosome and the lytic cycle induced (3a–4a). (B) Most mycobacteriophages rely on endolysin-holin systems to kill their hosts. Holins act as membrane proteins to help translocate the lysins to reach their targets: Lysin A degrades the peptidoglycan layer, whereas Lysin B cleaves the ester bonds between mycolic acids and the arabinogalactan in the mycolyl-arabinogalactan-peptidoglycan (mAGP) complex, disrupting the M. tuberculosis cell wall core (mAGP). Lysin B is also known to degrade trehalose dimycolates (TDMs) in the outer layer. Although lysis is the primary mycobacteriophage mechanism for bacterial death, secondary mechanisms such as the release of superoxide (O2−) radicals from lysed bacilli might also contribute in the elimination of M. tuberculosis.