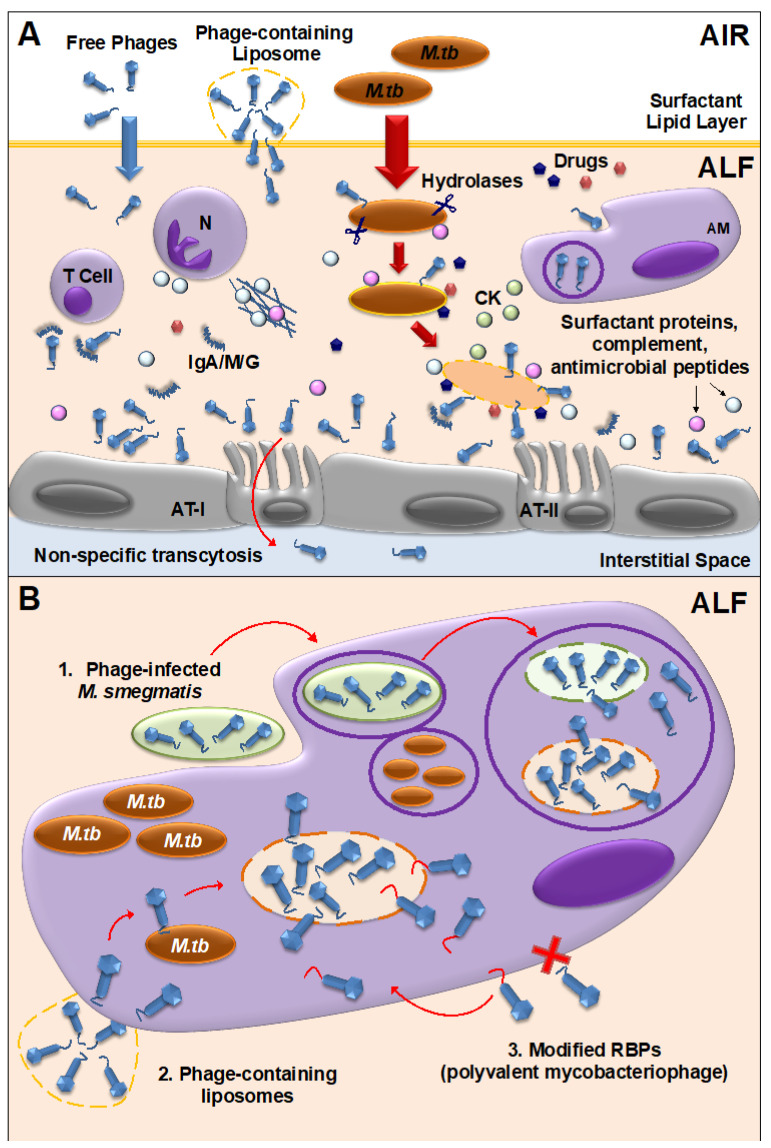
Figure 2.
Mycobacteriophage-M. tuberculosis interactions in the human lung alveolar environment. (A) Free and/or encapsulated mycobacteriophages (e.g., in liposomes) reach the lung alveolar space, cross the surfactant lipid layer and are bathed in alveolar lining fluid (ALF), where they can directly or indirectly interact with innate immune cells, generating a local immune response. Some phages will be cleared by phagocytic cells [e.g., alveolar macrophages (AMs)], or by neutralizing antibodies (IgA/M/G). If delivered in sufficient numbers, mycobacteriophages are capable of binding glycan residues in the mucus layer secreted by alveolar epithelial cells type II (AT-II) through Ig-like domains in their capsids, providing an extra non-host antimicrobial protection layer against M. tuberculosis infection. Mycobacteriophages can also be translocated to the interstitial space through the epithelium via a non-specific transcytosis mechanism. When M. tuberculosis enters the alveolar space, innate soluble molecules in ALF, including hydrolases (represented as scissors), modify the M. tuberculosis cell envelope, driving M. tuberculosis-host cells interactions. These ALF hydrolases-drive alterations will define de novo exposed M. tuberculosis surface receptors to be recognized by mycobacteriophages. Here is depicted a combined mycobacteriophage-drug therapy, where drugs and mycobacteriophages work together with an activated mammalian immune response [including neutrophil (N) degranulation and neutrophil extracellular traps [180] secretion, AMs activation, and cytokine and chemokine secretion] to kill M. tuberculosis and control the infection. (B) Different strategies can be used to deliver mycobacteriophages inside mammalian cells (e.g., AMs) to access intracellular M. tuberculosis: (1) Mycobacteriophage-infected M. smegmatis bacilli acting as vehicles are phagocytized by AMs being delivered into M. tuberculosis-containing phagosomes. Replicating mycobacteriophages within M. smegmatis lyse the bacterium accessing the phagosome lumen where they recognize, infect and subsequently lyse and kill M. tuberculosis; (2) Liposome-associated mycobacteriophages, which infect mammalian host cells more efficiently than free phages; (3) Generation of polyvalent mycobacteriophages through receptor binding protein (RBP) engineering to recognize mammalian immune cells in addition to receptors on the M. tuberculosis cell envelope surface. Note: This figure depicts a general overview of the mycobacteriophage-M.tb interactions in the human lung environment and with the mammalian immune system; and thus, does not fully represent the complexity of the processes described in the text.