Figure 1. Illustration of genetic circuitry enabling enhanced cyp51A expression in isolates possessing one of two representative azole resistance mechanisms.
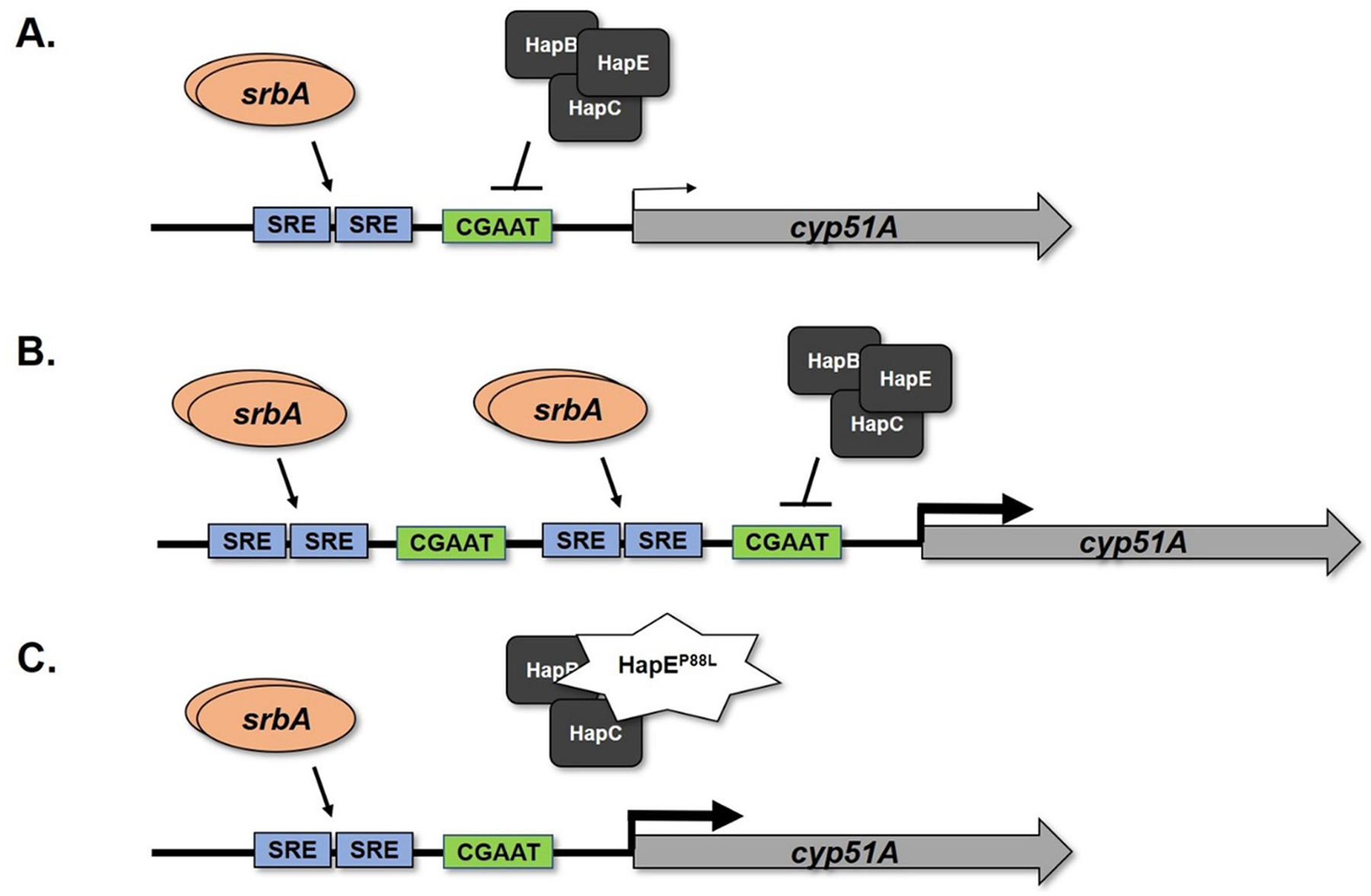
(A) The wild type configuration of the cyp51A promoter is shown. Under normal regulation, proper cyp51A expression levels are orchestrated by at least two transcriptional regulators. The activator, SrbA, binds to upstream sterol regulatory elements (SRE) to induce cyp51A expression, whereas the multi-subunit CCAAT-Binding Complex (CBC) binds to a conserved CGAAT motif downstream of the SREs to inhibit cyp51A expression. (B) Tandem repeat mutations of the cyp51A promoter wherein the SREs are duplicated, as occurs in isolates bearing the TR34 and TR46 mutations, lead to increased binding of SrbA and activation of gene expression. Although the CBC binding motif is also duplicated in these mutations, physical blockage of additional binding of the CBC complex occurs by the increased presence of the SrbA activator. (C) An illustration of a target gene-independent clinical triazole resistance mechanism involving a single mutation within the gene encoding a member of the heterotrimeric HAP complex, HapE. This mutation reduces binding of the entire CBC to its regulatory binding site in the cyp51A promoter. Note that, as in (B), reduced binding of the CBC complex results in loss of repression and consequently increased gene expression. Both representative mechanisms ultimately enable increased transcription of cyp51A mRNA, thus increasing the level of Cyp51A protein produced within fungi and enabling the biosynthesis of ergosterol.
