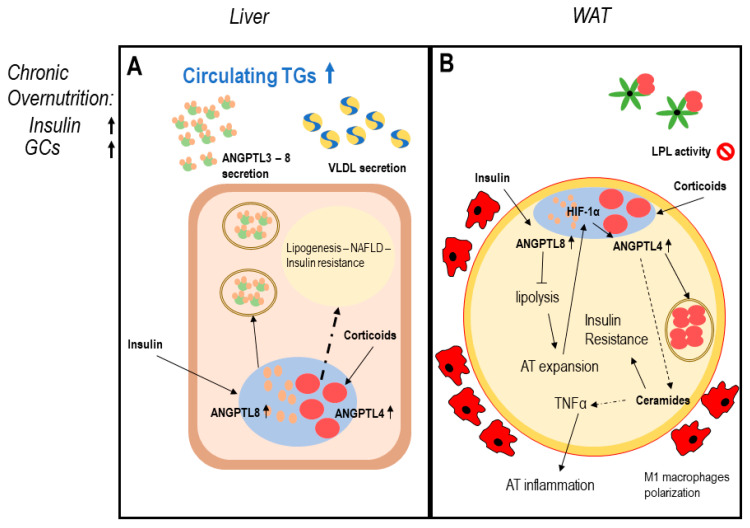
Figure 2.
ANGPTLs model of adipose tissue dysfunction. Legend: possible induction mechanism of adipose tissue dysfunction involving ANGPTL3-4-8. Dotted lines report data with a low level of evidence. Panel (A) describes dysregulation of hepatocytes function in conditions of chronic overnutrition: overexpressed insulin levels together with elevated plasma glucocorticoids in obese patients induce both ANGPTL8 and ANGPTL4 expression in hepatocytes [68,73,74,75,84,86,89,94]. ANGPTL4 possibly induces ceramides production; therefore, worsening hepatic insulin resistance and liver steatosis [35,81,89]. Panel (B) describes white adipose tissue (WAT) dysfunction in conditions of chronic overnutrition, potentially mediated by ANGPTLs system disruption: excess insulin induces overexpression of nuclear ANGPTL8 in WAT [67,68]. ANGPTL8 in turn enhances insulin signaling via AKT phosphorylation, chronic AKT phosphorylation induces worsening insulin resistance [73]. Permanent block in lipolysis leads to adipocyte mass expansion over oxygen diffusion capacity, this creates adipocyte stress and hypoxia-inducible factor 1-alpha (HIF1α) induction, which in turn induces ANGPTL4 expression [83,84], together with enhanced circulating glucocorticoids, typical of overnutrition. ANGPTL4 partly blocks WAT LPL, partly induces lipolysis and ceramide production [89]. Ceramide production is associated with worsening insulin resistance (IR) and inflammation, leading to WAT secretion of proinflammatory mediators, M1 macrophage infiltration, and adipocyte death typical of AT dysfunction [35,98].