Figure 5.
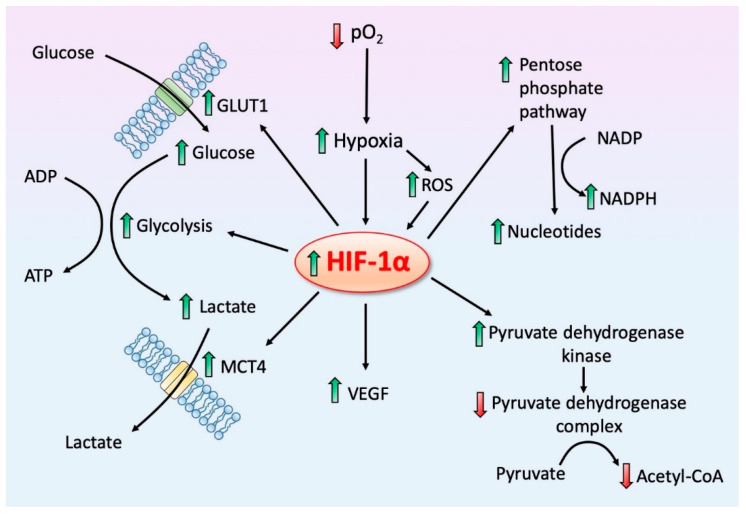
Hypoxia inducible factor-1 alpha (HIF-1α) is a central transcription agent in mediating Warburg-type metabolism within diseased cells. HIF-1α is part of an oxygen sensing system which becomes activated when the partial pressure (pO2) of intracellular oxygen becomes depressed, that is, when cells become hypoxic. A primary means by which HIF-1α promotes Warburg-type metabolism is by upregulating the mitochondrial enzyme pyruvate dehydrogenase kinase which, in turn, inhibits pyruvate dehydrogenase complex thereby reducing the conversion of pyruvate to acetyl coenzyme A in the mitochondria. The reduction of acetyl coenzyme A in the mitochondrial severely restricts the intramitochondrial synthesis of melatonin (see Figure 2). Further, HIF-1α promotes the pentose phosphate pathway (see Figure 4) and upregulates glycolysis by stimulating the glucose transporter (GLUT1), which accelerates the influx of glucose (the “sweet tooth” phenomenon), and promoting the efflux of lactate by stimulating the monocarboxylate transporter 4 (MCT4) which also stimulates neovasculogenesis by upregulating vascular endothelial growth factor (VEGF). Melatonin, by directly or indirectly inhibiting HIF-1α, reverses the Warburg effect and prevents the associated metabolic activities. Reactive oxygen species, which are produced more abundantly under hypoxic conditions, aid in the stabilization of HIF-1α.