Abstract
Background
Because the reliability of repetitive transcranial magnetic stimulation (rTMS) in treating poststroke cognitive impairment has not been convincingly demonstrated, we systematically examined the effectiveness of this regimen with 2 protocols.
Methods
We randomly allocated 41 patients with poststroke cognitive impairment to receive 5 Hz rTMS (n = 11), intermittent theta burst stimulation (iTBS; n = 15) or sham stimulation (n = 15). Each group received 10 stimulation sessions over the left dorsolateral prefrontal cortex. We performed the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and the Beck Depression Inventory at baseline and after the intervention.
Results
The 5 Hz rTMS group showed significantly greater improvement than the sham group in RBANS total score (p = 0.006), attention (p = 0.001) and delayed memory (p < 0.001). The iTBS group showed significantly greater improvement than the sham group in RBANS total score (p = 0.005) and delayed memory (p = 0.007). The 5 Hz rTMS group exhibited a superior modulating effect in attention compared to the iTBS group (p = 0.016). Patients without comorbid hypertension (p = 0.008) were predisposed to favourable therapeutic outcomes.
Limitations
Although we included only patients with left hemispheric stroke, heterogeneity associated with cortical and subcortical implications existed. We did not investigate the remote effects of rTMS.
Conclusion
Our results demonstrated that both 5 Hz rTMS and iTBS were effective for poststroke cognitive impairment in terms of global cognition, attention and memory function; the domain of attention was susceptible to 5 Hz modulation. Treatment with 5 Hz rTMS may slow cognitive decline, representing both a pivotal process in poststroke cognitive impairment and an aspect of neuroplasticity that contributes to disease-modifying strategies.
Clinical trial registration
Introduction
Poststroke cognitive impairment (PSCI) is one of the most detrimental neurologic conditions, and it has a high prevalence, affecting about 30% to 40% of stroke survivors.1,2 It presents in several domains, including attention, memory, language and visuospatial function, considerably compromising patients’ quality of life and increasing health care costs.2 However, the pathophysiology of PSCI remains unclear; the mechanism of PSCI may be related to the stroke itself, or it may be a result of stroke-related aggravation of preexisting vascular risk factors, white matter changes or associated degenerative pathology.3 Decreased levels of neurotransmitters, including acetylcholine and dopamine, have also been noted in patients with PSCI4 and in those with vascular cognitive impairment.5,6
Some specific pharmacological therapies, such as cholinesterase inhibitors, may have partial positive effects,7 but meta-analyses of nonpharmacological approaches such as psychosocial rehabilitation have shown unclear results.3,8 Effective and optimal treatments for PSCI are needed.
Repetitive transcranial magnetic stimulation (rTMS), a painless noninvasive technology, has been widely used in stroke patients to improve motor dysfunction, aphasia, dysphagia and visuospatial neglect. The use of rTMS to treat cognitive disorders such as normal brain aging, Alzheimer disease and Parkinson disease has drawn considerable attention, but only a few small-scale studies have investigated the effects of rTMS on PSCI. Rektorova and colleagues9 enrolled patients with cerebrovascular disorders and applied 10 Hz rTMS over the left dorsolateral prefrontal cortex (dlPFC); they reported mild but significant effects in executive function after stimulation. Park and colleagues10 administered 10 Hz rTMS to the left dlPFC in stroke patients and reported significant improvement in global cognitive function after the intervention. Kim and colleagues11 administered various rTMS protocols in stroke patients with cognitive deficit, but they did not detect any measurable effects on cognition. In all of these studies, preliminary results were confounded by poor patient stratification or a lack of sham control. Firm conclusions regarding the effects of rTMS on PSCI drawn from a formal investigation are needed.
Intermittent theta burst stimulation (iTBS) over the left dlPFC is a novel but theorized modality that provides neuropsychiatric enhancement for patients with depressive disorders, autism12,13 and Parkinson disease with mild cognitive impairment.14 The 3-day iTBS protocol resulted in significant improvements in overall cognition, attention and visuospatial domains in patients with Parkinson disease.14 These effects were likely mediated by the association of the dlPFC with the caudate nucleus (dlPFC striatal link) and an increase in dopamine release after modulation of the left dlPFC.15 Although research has explored the effectiveness of iTBS in the treatment of Parkinsonism with cognitive impairment, the effects of its application in patients with PSCI remain unclear. Moreover, the effectiveness of conventional high-frequency rTMS and iTBS on mood control in healthy people and patients with depression remains controversial. Patients with depression exhibit different pathophysiological peculiarities from those who have vascular dementia or PSCI, including a significant interhemispheric discrepancy in motor cortex excitability, imbalanced intracortical neurochemical circuitry and impaired neuroplasticity to neuromodulation.16 Moreover, motor cortex hyperexcitability has been noted in patients with vascular dementia.17 Therefore, the effects of both paradigms on poststroke depression merit further investigation. Neurophysiologically, iTBS may have an equal or greater excitatory effect compared to conventional rTMS.18,19 Studies on depression treatment have reported that the effectiveness of iTBS is similar to that of high-frequency rTMS.20 However, in rat models, conventional high-frequency rTMS over the motor cortex led to better neurogenesis promotion than iTBS.21 In another study applying rTMS to healthy people, high-frequency rTMS produced a larger response than iTBS.22 The excitatory and inhibitory peculiarities of motor cortex theta burst stimulation might not be transferable to prefrontal theta burst stimulation. Consensus on the effectiveness of conventional high-frequency rTMS or iTBS in neuropsychiatric treatment is lacking; the optimal PSCI treatment protocol requires investigation.
Because conventional high-frequency rTMS and iTBS could promote local neurogenesis, increase cortical connectivity and enhance neuroplasticity, we hypothesized that both modalities would facilitate improvement in PSCI. In the current study, we selected 5 Hz as the frequency for conventional rTMS; this frequency has been applied to optimize the modulation of working memory.23 This randomized, controlled study was aimed at investigating the effects on PSCI of 5 Hz rTMS and iTBS over the left dlPFC in patients with left hemispheric stroke and compared the effects of the 2 modalities on patients’ global, memory, attention, language and visuospatial cognitive function.
Methods
Participants
We surveyed stroke patients with cognitive impairment who visited the rehabilitation clinic of a tertiary medical centre or were admitted to its stroke ward. Inclusion criteria were as follows: left hemispheric ischemic or hemorrhagic stroke more than 3 months previously with cognitive impairment, defined by a Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)24 score below 85; no seizure history; no intracranial occupying lesion, including arteriovenous malformation or brain tumour, according to imaging results; and no concurrent use of antidepressants or neurostimulators. Exclusion criteria included unstable cardiac dysrhythmia, fever, infection, hyperglycemia, epilepsy or previous administration of tranquilizers, neurostimulators or other medication that significantly affected the cortical motor threshold.25 We excluded patients with metallic intracranial devices, pacemakers or other electronic devices in their bodies. The rTMS protocols used in the current study were in accordance with the safety guidelines for rTMS applications.26
Of the 65 patients we surveyed, 45 fulfilled the criteria, 20 did not and 1 declined participation. This study was approved by the institutional review board of Taipei Veterans General Hospital (RCT02006615), and all patients provided written informed consent before participation.
Design
This was a randomized, controlled, double-blind study. Randomization order was computer generated and concealed in sequentially numbered opaque envelopes by an independent statistician. The 44 patients were randomly assigned to 5 Hz rTMS (n = 14), iTBS (n = 15) and sham (n = 15) groups equally. In each participant, sham or actual stimulation was applied over the left dlPFC in 10 sessions over 10 consecutive weekdays.
Determining resting motor threshold
We applied stimulation using the Rapid2 (Magstim) and a 70 mm figure-8 coil. Patients were asked to sit relaxed in a chair with their eyes open. The coil was systematically displaced (mapping) over the primary motor cortex until the largest consistent motor evoked potential response from the contralateral first dorsal interosseous was recorded. The resting motor threshold for the first dorsal interosseous, as the minimal intensity, was that at which a motor evoked potential of at least 50 mV could be elicited in 5 of 10 consecutive sessions. 27 We connected a Keypoint electromyograph machine (Dantec) to the TMS stimulator to record the motor evoked potential signals of the first dorsal interosseous muscle, and the amplified (100–1 mV/div) and bandpass-filtered (20–2000 Hz) signals were digitized at a sampling rate of 20 kHz.
Stimulation protocol
Each patient received 10 days of rTMS treatment, administered in the morning from Monday to Friday for 2 consecutive weeks. The left dlPFC (F3 point) was stimulated according to the international 10/20 electroencephalography (EEG) recording system to stimulate the left prefrontal cortex.11 The intensity for the 5 Hz rTMS and iTBS groups was set at 80% of the resting motor threshold.11,14,28
The iTBS treatment consisted of 3 pulses of 50 Hz bursts repeated at 5 Hz (2 s on and 8 s off) for a total of 190 seconds (600 pulses). The 5 Hz rTMS protocol was applied at an intensity of 80% of the resting motor threshold, with 2 s trains (10 pulses) at an intertrain interval of 8 seconds, repeated every 10 seconds for a total of 10 minutes (600 pulses). The sham condition involved similar procedures, except that a sham coil was used. We used a placebo coil (Magstim) for the sham stimulation, which delivered less than 5% of the magnetic output with an audible click on discharge. Because none of the patients had experienced rTMS, they did not know whether they were receiving real or sham rTMS.
Assessment of cognitive and depression status
A therapist blinded to group allocation evaluated the cognitive and depression status of all patients before the first intervention session and 1 day after completion of the protocol using the RBANS and Beck Depression Inventory (BDI).29
The RBANS generates 5 index scores for 5 neurocognitive domains: immediate memory, visuospatial and constructional abilities, language, attention and delayed memory. The total score is calculated by summarizing these 5 index scores, and the cutoff for the average score is 90 to 109; the lower the total RBANS score, the poorer the cognitive function. For BDI scores, we used standard cutoff values: 1 to 10, normal; 11 to 16, mild mood disturbance; 17 to 27, moderate depression; 21 to 30, moderate depression; 31 to 40, severe depression; and 41 to 63, extreme depression.
Statistical analysis
We compared baseline assessments and biographic data between groups using 1-way analysis of variance for continuous data and χ2 tests for categorical data, as appropriate. To determine improvements in RBANS scores, we used the Wilcoxon signed rank test for intragroup comparison. We used linear regression analysis for intergroup and subgroup comparisons (presence or absence of comorbidity) of changes in RBANS and BDI scores, with age, time since stroke onset, language and education level as explanatory factors. To determine the associations between clinical outcomes and the demographic data and baseline scores, we subjected changes in RBANS total and subtest scores to multiple regression analysis with age, time since stroke onset, language score in RBANS and education level as explanatory factors. The level of significance was set at p < 0.05. We conducted these analyses using SPSS version 22. Using G-POWER software to calculate sample size, we determined that the desired sample size was 9 based on assumptions of an α of 0.05, a power of 0.8 and an effect size of 1.18.
Results
Demographic and clinical characteristics
Table 1 lists patients’ demographic and clinical data. Three patients in the 5 Hz rTMS group withdrew from the intervention sessions because of commuting difficulties, resulting in a total of 11 patients for this group. The initial RBANS scores in the 3 groups indicated severe cognitive impairment, and baseline BDI scores revealed normal to mild mood disturbances. The mean group data for all cognitive assessments are summarized in Table 1. At baseline, the 3 groups demonstrated no significant differences in age, sex, lesion site (cortex or not), education level, or total and index RBANS scores. Comparisons of initial demographic characteristics between groups revealed a significantly longer disease duration in the 5 Hz group at baseline (p = 0.016); we used this as an explanatory variable for intergroup comparison of outcome measures. During the experimental period, patients demonstrated no seizure or other adverse effects.
Table 1.
Demographic and clinical characteristics*
| Characteristic | iTBS (n = 15) | 5 Hz rTMS (n = 11) | Sham (n = 15) | p value |
|---|---|---|---|---|
| Age, yr | 60.13 ± 14.1 | 57.45 ± 12.3 | 56.23 ± 12 | 0.77 |
| M/F, n | 11/4 | 9/2 | 13/2 | 0.65 |
| Education, yr | 11.27 ± 5.4 | 14 ± 2.8 | 13.64 ± 1.9 | 0.56 |
| Poststroke duration, mo | 18.47 ± 20.21 | 33.27 ± 26.4 | 38 ± 7.9 | 0.016† |
| Ischemic stroke/hemorrhagic stroke, n | 7/8 | 3/8 | 10/5 | 0.14 |
| Lesion site (cortex + subcortical, or subcortical), n | 11/4 | 10/1 | 12/3 | 0.31 |
| Anomic aphasia, n | 5 | < 5 | < 5 | 0.86 |
| Comorbidities, n | ||||
| Hypertension | 9 | < 5 | 10 | 0.29 |
| Diabetes mellitus | 5 | < 5 | < 5 | 0.23 |
| Dyslipidemia | < 5 | 0 | < 5 | 0.43 |
| RBANS scores | ||||
| Total | 73.7 ± 12.2 | 69.1 ± 10.9 | 65.3 ± 16.1 | 0.19 |
| Immediate memory | 73.1 ± 16.6 | 69.2 ± 20.0 | 61.3 ± 20.0 | 0.25 |
| Visuospatial ability | 98.9 ± 13.4 | 87.0 ± 13.9 | 82.9 ± 15.6 | 0.17 |
| Language | 70.1 ± 14.0 | 76.3 ± 4.8 | 63.3 ± 18.1 | 0.25 |
| Attention | 77.5 ± 19.1 | 70.1 ± 18.9 | 76.1 ± 20.8 | 0.52 |
| Delayed memory | 79.5 ± 18.8 | 75.1 ± 21.1 | 76.1 ± 20.8 | 0.86 |
| Beck Depression Inventory score | 14.5 ± 10.0 | 13.0 ± 13.2 | 9.2 ± 8.2 | 0.27 |
F = female; iTBS = intermittent theta burst stimulation; M = male; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; rTMS = repetitive transcranial magnetic stimulation.
Data are mean ± standard deviation unless otherwise specified. Populations of fewer than 5 people have been rounded to protect participant privacy.
One-way analysis of variance for continuous data and a χ2 test, with significance levels set at p < 0.05. Post hoc analyses revealed significantly longer disease duration in the 5 Hz rTMS group at baseline (p = 0.016), which we used as an explanatory variable for intergroup comparison. Bonferroni correction: iTBS v. 5 Hz rTMS, Z = −3.776, p = 0.001.
Group-wise improvement
After the 10 stimulation treatment sessions, the 5 Hz rTMS group exhibited significant increases in total RBANS score (p = 0.003) and improvements in delayed memory (p = 0.007) and attention (p = 0.005) compared with baseline. Similarly, the iTBS group demonstrated significant increases in total RBANS score (p = 0.001) and improvements in immediate memory (p = 0.006), language (p = 0.005) and delayed memory (p = 0.008). We noted no significant differences in BDI scores before and after stimulation in any of the 3 groups. Results are summarized in Table 2.
Table 2.
Mean RBANS and Beck Depression Inventory scores by group*
| Group | Pre-rTMS | Post-rTMS | Difference | |Z| | p value | Power |
|---|---|---|---|---|---|---|
| iTBS | ||||||
| RBANS scores | ||||||
| Total | 73.7 ± 12.2 | 80.1 ± 16.0 | 6.4 ± 6.2 | 3.327 | 0.001 | 0.95 |
| Immediate memory | 73.1 ± 16.5 | 81.1 ± 20.9 | 8 ± 9.6 | 2.737 | 0.006 | |
| Visuospatial ability | 98.9 ± 13.4 | 100.2 ± 14.2 | 1.3 ± 3.2 | 1.461 | 0.14 | |
| Language | 70.1 ± 14.0 | 76.1 ± 15.6 | 6 ± 8.7 | 2.807 | 0.005 | 0.68 |
| Attention | 77.5 ± 19.1 | 80.1 ± 17.1 | 2.7 ± 7.8 | 1.289 | 0.20 | |
| Delayed memory | 79.5 ± 18.8 | 84.6 ± 19.8 | 5.1 ± 9.0 | 2.67 | 0.008 | 0.51 |
| Beck Depression Inventory score | 14.5 ± 10.0 | 14.1 ± 9.5 | −0.4 ± 0.8 | 1.732 | 0.08 | |
| 5 Hz rTMS | ||||||
| RBANS scores | ||||||
| Total | 69.1 ± 10.9 | 76.9 ± 10.6 | 7.8 ± 5 | 2.938 | 0.003 | 0.99 |
| Immediate memory | 69.2 ± 20.0 | 75.4 ± 21.5 | 6.2 ± 11.7 | 1.602 | 0.11 | |
| Visuospatial ability | 87.0 ± 13.9 | 91.0 ± 16.8 | 4 ± 12.6 | 0.889 | 0.37 | |
| Language | 76.3 ± 4.8 | 77.7 ± 7.3 | 1.5 ± 8.8 | 0.954 | 0.34 | |
| Attention | 70.1 ± 19.0 | 84.6 ± 15.1 | 14.5 ± 15.1 | 2.805 | 0.005 | 0.41 |
| Delayed memory | 75.1 ± 21.1 | 90.6 ± 16.0 | 15.5 ± 13 | 2.713 | 0.007 | 0.93 |
| Beck Depression Inventory score | 13.0 ± 13.2 | 8.3 ± 6.5 | −4.7 ± 11.4 | 0.949 | 0.34 | |
| Sham | ||||||
| RBANS scores | ||||||
| Total | 65.3 ± 16.1 | 65.9 ± 16.1 | 0.6 ± 4.7 | 0.000 | 1.000 | |
| Immediate memory | 61.3 ± 20.0 | 64.1 ± 23.7 | 2.7 ± 7.9 | 1.263 | 0.21 | |
| Visuospatial ability | 82.9 ± 15.6 | 80.1 ± 12.2 | −2.8 ± 10.9 | 0.756 | 0.45 | |
| Language | 63.3 ± 18.1 | 62.7 ± 17.5 | −0.6 ± 5.7 | 0.119 | 0.91 | |
| Attention | 76.1 ± 20.8 | 74.5 ± 22.6 | −1.6 ± 7.3 | 0.77 | 0.44 | |
| Delayed memory | 76.1 ± 20.8 | 67.4 ± 23.1 | −8.7 ± 15.4 | 1.923 | 0.054 | |
| Beck Depression Inventory score | 9.2 ± 8.2 | 7.8 ± 7.6 | −1.1 ± 2.2 | 1.802 | 0.07 | |
iTBS = intermittent theta burst stimulation; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; rTMS = repetitive transcranial magnetic stimulation.
Values are expressed as mean ± standard deviation. Wilcoxon signed rank test for intragroup comparison; improvement compared with baseline. Significance levels set at p < 0.05.
Intergroup comparison
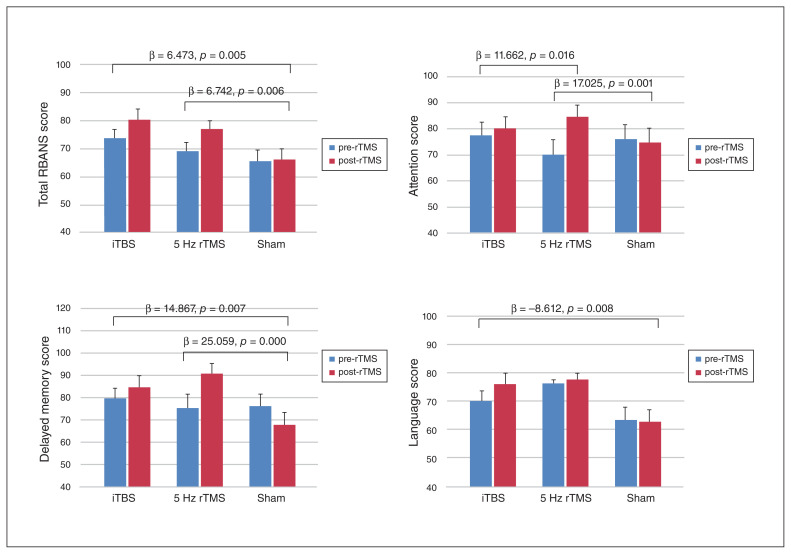
Compared with the sham group, both the 5 Hz rTMS (p = 0.006) and iTBS (p = 0.005) groups demonstrated significant increases in poststimulation total RBANS scores after age, time since stroke onset, language and education level were entered as explanatory factors (Fig. 1). Moreover, compared with the sham group, we noted significant improvement in the domains of attention (p = 0.001) and delayed memory (p < 0.001) in the 5 Hz rTMS group. Attention was the only domain that showed significant differences between the 5 Hz rTMS and iTBS groups: 5 Hz rTMS yielded superior conditioning efficacy in the attention domain compared with iTBS (p = 0.016), with corrected covariances of age, time since stroke onset, language and education level.
Fig. 1.
Mean group values with standard deviations for Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores and subtest scores. Statistical analysis through linear regression with age, time since stroke onset, language and education level as explanatory factors. Significant differences between groups were set at p < 0.05. iTBS = intermittent theta burst stimulation; rTMS = repetitive transcranial magnetic stimulation.
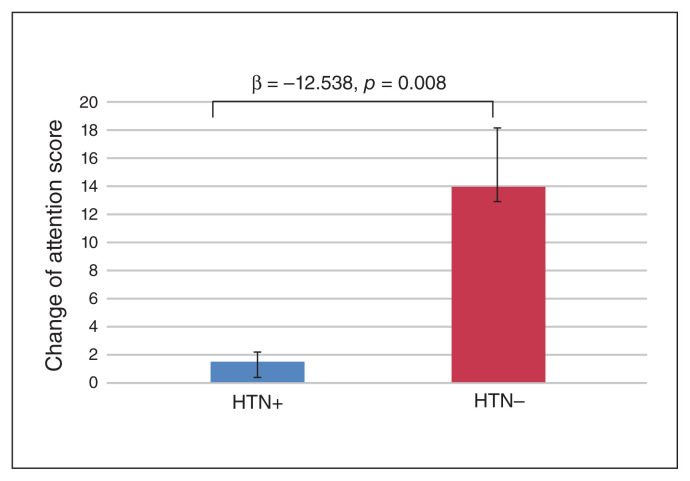
Comorbid hypertension yielded a negative effect on attention improvement (β = −12.538, p = 0.008; Fig. 2) when analyzing patients in the experimental groups.
Fig. 2.
Subgroup analyses revealed that patients with hypertension (HTN+) yielded lower attention improvement (p = 0.008) than patients with no history of hypertension (HTN−). Statistical analysis through linear regression with age, time since stroke onset, language and education level as explanatory factors. Significant differences between groups were set at p < 0.05.
We noted no significant differences in BDI scores among the 3 groups.
Correlation analyses
The effects of rTMS on all patients as measured by RBANS total and subtest improvements were not correlated with the demographic data (age, sex, time since stroke onset, education level, or presence of diabetes mellitus or hyperlipidemia), except for comorbid hypertension (β = −12.48, p = 0.01). Hypertension had a negative effect on attention improvement for rTMS modulation.
Discussion
This randomized, controlled, double-blind study provided the first evidence for the therapeutic potential of 2 TMS protocols for PSCI. Using a left hemispheric stroke model, we noted that both iTBS and 5 Hz rTMS improved global cognition and memory function without altering mood status. Both 5 Hz rTMS and iTBS were effective and safe modulating protocols for treating patients with left brain stroke and PSCI. Notably, 5 Hz rTMS yielded a superior modulating effect on attention compared to iTBS. Patients without comorbid hypertension were more susceptible to rTMS modulation for cognitive function. Recently, mild cognitive impairment after cerebrovascular lesion has been identified as a key target for therapeutic strategies to slow the progression of cognitive decline and prevent such decline from leading to vascular dementia or mixed Alzheimer disease.30 The modulating effect of rTMS on cognitive restoration makes it possible to hinder or delay the decline of mental condition after stroke; this finding provides further support for the role of noninvasive brain stimulation techniques as possible disease modifiers in PSCI.
The mechanism underlying rTMS-related global cognitive improvement remains unclear. Stimulation may have an immediate and direct effect on the target area, affecting the interneurons and increasing blood flow and levels of neurotransmitters such as acetylcholine, dopamine, norepinephrine and serotonin.31,32 Moreover, rTMS may also act by modulating the efficacy of the synapses (e.g., increasing the synaptic strength) through long-term potentiation.33,34 These facilitatory after-effects of the cortical excitability of rTMS are associated with increased γ-aminobutyric acid (GABA)–mediated inhibition and upregulated N-methyl-d-aspartate receptor activity,32 particularly after multiple rTMS sessions.35 Because memory and learning processes are regulated by synaptic neuronal activities, promoting the synaptic strength through rTMS may improve memory and learning.36 Notably, the dlPFC is a region in which a reduction in GABA levels is associated with aging-related cognitive decline.37
Previous studies have reported that conventional highfrequency rTMS over the left dlPFC may improve global cognition in elderly people, patients with dementia and patients with Alzheimer disease.38,39 However, studies have reported conflicting results for the use of conventional rTMS in patients with PSCI.9,11 This could be because of differences in stimulation protocols and mixed groupings comprising different lesion sites. Moreover, results have indicated that iTBS in healthy individuals40 and those with Parkinson disease and cognitive impairment yields effects with varying degrees of reliability.14 Our results revealed that both 5 Hz rTMS and iTBS over the dlPFC improved memory function. In the RBANS, immediate memory testing includes list learning and story memory, and delayed memory testing includes list recall, list recognition, story recall and figure recall. Both immediate memory and delayed memory are parts of working memory, which is not only a form of short-term memory but also a series of interactive processes that allow for the manipulation of information and use of learned information. 41 Our findings were consistent with those of previous studies: that patients with left hemispheric stroke may sustain significant impairments in immediate recall and delayed recall,42,43 which can be alleviated with conventional high-frequency rTMS over the left dlPFC.9,44 The mechanism underlying this effect may be associated with changes in the interconnected regions and neurotransmitter levels elicited by rTMS, because the left dlPFC contains networks that are crucial for working memory32,45 and are partially attributable to increased GABA levels37 and acetylcholine inputs into the hippocampus after modulation.46
Our results indicated that 5 Hz rTMS over the left dlPFC enhanced attention in PSCI patients — in line with previous studies applying high-frequency rTMS over the left dlPFC in healthy people and patients with depression.47,48 From the molecular perspective, the dopamine system is crucial in attention modification. In stroke patients with brain damage or cerebral ischemia, which may strongly affect dopaminergic neurotransmission,4 conventional high-frequency rTMS over the left dlPFC could effectively modulate dopamine release. 49 Some studies have used conventional high-frequency rTMS to modulate PSCI, but studies using iTBS to modulate PSCI are lacking. No specific difference in efficacy has been reported for 10 Hz rTMS and iTBS in terms of cortical excitability, motor performance50 or mood status.18 However, in the current study, the attention-modulating effect of 5 Hz rTMS was superior to that of iTBS. Luber and colleagues23 observed that 5 Hz rTMS may be superior to other types of rTMS (1 Hz and 20 Hz) in enhancing working memory, which is specific to the timing of stimulation relative to task performance. Yamanaka and colleagues51 demonstrated that application of 5 Hz rTMS in the parietal area could reduce the reaction time of working memory. A study investigating the effect of iTBS on panic disorder associated with Parkinson disease suggested that iTBS failed to normalize or enhance the prefrontal hypoactivation during cognitive performance. Instead, the patients in the sham group presented with augmented prefrontal neuroactivity.52 Li and colleagues53 used electroencephalographic recordings as the basis for investigating the efficacy of 5 Hz rTMS in enhancing working memory in healthy people. Following stimulation, θ-band oscillations were significantly increased, and α-band oscillations were significantly decreased; moreover, these results were significantly correlated with an improvement in working memory.53 Another study investigated how cortical oscillations are modulated by various rTMS protocols over the left dlPFC and compared the spectral power of EEG following stimulation.54 The authors observed that the iTBS protocol was associated with decreased EEG power in the θ band and, conversely, increased power in high-frequency β and γ bands. Although no study has directly compared 5 Hz and iTBS protocols, the after-effects of the protocols differ in evaluations of cortical oscillation, namely in terms of high- or low-frequency band power; such differences may correspond to dissimilar modulating effects on working memory and attention. Moreover, the relatively longer conditioning duration of 5 Hz rTMS compared with that of iTBS contributed to the increased effective retention process and top–down attention control.
The prevailing view is that hypertension is one of the principal risk factors for cerebral small vessel disease, neurodegenerative disease and cognitive impairment.55 The progression of white matter hyperintensity is associated with decreased mobility and cognitive function in elderly people.56 Among the genetic, metabolic and vascular risk factors, systemic hypertension has proven to be a relatively strong indicator of the progression of small vessel disease, which may also negatively affect the efficacy of rTMS modulation.57 Cheeran and colleagues58 noted that participants with different polymorphisms of the BDNF gene had different levels of susceptibility to rTMS modulation for long-term neuroplasticity. Such subcortical microangiopathy and related encephalopathy may impair corticospinal tract integrity, further compromising the therapeutic efficacy of modulation. The findings of these studies, linking hypertensive neurotrophic dysfunction with the inferior modulating effect of rTMS conditioning, indicate that these neurovascular mechanisms may be underlying factors in the decreased response of hypertensive patients to rTMS manipulation.
In the current study, we noted no significant improvement in the visuospatial domain after applying high-frequency rTMS over the left dlPFC, probably because left hemispheric stroke does not impair visuospatial function in the way that right hemispheric stroke does.59 The visuospatial scores of our patients were mostly in the range of 80 to 100, representing a low to average score compared to the healthy population. Hence, after stimulation, we noted no reserve capacity for visuospatial improvement. Moreover, we did not observe significant improvement in mood status after stimulation, probably because our patients had normal to mild mood disturbance at baseline, leaving little potential or requirement for further improvement of mood status after rTMS conditioning. This result also indicated that promotion of cognitive function resulted from the effects of rTMS, not from the alteration of depression.
Limitations
The present study had several limitations. First, although we included only patients with left hemispheric stroke, there was heterogeneity associated with cortical and subcortical implications. A stratification of patients on the basis of the integrity of clinical, biological, functional, neuroimaging and neurophysiological biomarkers is probably the optimal method for improving outcome measures and achieving more accurate predictive diagnoses.28 Second, we used the RBANS as our evaluation tool, and it could not analyze other cognitive domains such as executive function and long-term memory. The mechanism of rTMS remains largely unknown; remote effects of rTMS based on microstructural changes can be evaluated using a combination of advanced techniques, such as EEG and functional MRI. Further investigation using larger homogenous samples, more comprehensive cognitive evaluations and a combination of EEG and MRI is warranted to describe short- and long-term recovery from PSCI. Studies on right hemispheric stroke could be useful in exploring the effects of rTMS on PSCI, particularly on the impaired visuospatial domain. Further studies are needed to explore the potential of this modality administered in multiple courses for a long period to slow cognitive decline in patients with PSCI.
Conclusion
This randomized, controlled, double-blind study provided promising results for the application of TMS over the left dlPFC of patients with PSCI. We confirmed the beneficial effects of 2 excitatory TMS paradigms: 5 Hz rTMS and iTBS. We demonstrated that global cognition and memory function could be enhanced using 5 Hz rTMS and iTBS, and that attention in particular could be augmented through 5 Hz rTMS. Because PSCI is a dynamic condition based on clinical and pathophysiological observation and is a risk factor for the future development of dementia, it should be the target of preventive strategies. We propose that this novel intervention may be applied as an effective and safe complementary therapy that contributes to establishing a disease-modification strategy for PSCI.
Acknowledgments
The authors thank Hsin-Yi Huang for her contribution to this research.
Footnotes
Funding: This work was supported by the Taipei Veterans General Hospital Grant (V104C058).
Competing interests: None declared.
Contributors: P. Tsai designed the study. W. Lin, C. Kao, K. Tsai and P. Lin acquired the data, which P. Tsai and P. Lin analyzed. P. Tsai, C. Kao and K. Tsai wrote the article, which W. Lin, K. Tsai, and P. Lin reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Leys D, Hénon H, Mackowiak-Cordoliani M-A, et al. Poststroke dementia. Lancet Neurol. 2005;4:752–9. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 2.Hu G-C, Chen Y-M. Post-stroke dementia: epidemiology, mechanisms and management. Int J Gerontol. 2017;11:210–4. [Google Scholar]
- 3.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. 2016;12:105. doi: 10.2147/VHRM.S75306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartolo M, Zucchella C, Capone A, et al. An explorative study regarding the effect of l-deprenyl on cognitive and functional recovery in patients after stroke. J Neurol Sci. 2015;349:117–23. doi: 10.1016/j.jns.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Bella R, Cantone M, Lanza G, et al. Cholinergic circuitry functioning in patients with vascular cognitive impairment — no dementia. Brain Stimul. 2016;9:225–33. doi: 10.1016/j.brs.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Lanza G, Bramanti P, Cantone M, et al. Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav Neurol. 2017 doi: 10.1155/2017/1421326. 1421326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooq MU, Min J, Goshgarian C, et al. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31:759–76. doi: 10.1007/s40263-017-0459-3. [DOI] [PubMed] [Google Scholar]
- 8.Elliott M, Parente F. Efficacy of memory rehabilitation therapy: a meta-analysis of TBI and stroke cognitive rehabilitation literature. Brain Inj. 2014;28:1610–6. doi: 10.3109/02699052.2014.934921. [DOI] [PubMed] [Google Scholar]
- 9.Rektorova I, Megova S, Bares M, et al. Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci. 2005;229–30:157–61. doi: 10.1016/j.jns.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Park I-S, Yoon J-G. The effect of computer-assisted cognitive rehabilitation and repetitive transcranial magnetic stimulation on cognitive function for stroke patients. J Phys Ther Sci. 2015;27:773–6. doi: 10.1589/jpts.27.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BR, Kim D-Y, Chun MH, et al. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil. 2010;89:362–8. doi: 10.1097/PHM.0b013e3181d8a5b1. [DOI] [PubMed] [Google Scholar]
- 12.Ni H-C, Hung J, Wu C-T, et al. The impact of single session intermittent theta-burst stimulation over the dorsolateral prefrontal cortex and posterior superior temporal sulcus on adults with autism spectrum disorder. Front Neurosci. 2017;11:255. doi: 10.3389/fnins.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C-T, Chen M-H, Juan C-H, et al. Effects of prefrontal theta-burst stimulation on brain function in treatment-resistant depression: a randomized sham-controlled neuroimaging study. Brain Stimul. 2018;11:1054–62. doi: 10.1016/j.brs.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Trung J, Hanganu A, Jobert S, et al. Transcranial magnetic stimulation improves cognition over time in Parkinson’s disease. Parkinsonism Relat Disord. 2019;66:3–8. doi: 10.1016/j.parkreldis.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Ko JH, Monchi O, Ptito A, et al. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task– a TMS–[11C]raclopride PET study. Eur J Neurosci. 2008;28:2147–55. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantone M, Bramanti A, Lanza G, et al. Cortical plasticity in depression: a neurochemical perspective from transcranial magnetic stimulation. ASN Neuro. 2017;9 doi: 10.1177/1759091417711512. 1759091417711512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennisi G, Bella R, Lanza G. Motor cortex plasticity in subcortical ischemic vascular dementia: What can TMS say? Clin Neurophysiol. 2015;126:851–2. doi: 10.1016/j.clinph.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Bakker N, Shahab S, Giacobbe P, et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015;8:208–15. doi: 10.1016/j.brs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Di Lazzaro V, Dileone M, Pilato F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–6. doi: 10.1152/jn.00781.2010. [DOI] [PubMed] [Google Scholar]
- 20.Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Zheng H, Zhang L, et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017;18:455. doi: 10.3390/ijms18020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin A, Sun J, Ayaz H, et al. Evaluation of evoked responses to pulse-matched high frequency and intermittent theta burst transcranial magnetic stimulation using simultaneous functional near-infrared spectroscopy. Neurophotonics. 2017;4 doi: 10.1117/1.NPh.4.4.041405. 041405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luber B, Kinnunen L, Rakitin B, et al. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency-and time-dependent effects. Brain Res. 2007;1128:120–9. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exper Neuropsychol. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 25.Paulus W, Classen J, Cohen LG, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–63. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shajahan PM, Glabus MF, Steele JD, et al. Left dorso-lateral repetitive transcranial magnetic stimulation affects cortical excitability and functional connectivity, but does not impair cognition in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:945–54. doi: 10.1016/s0278-5846(02)00210-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35:416–31. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- 30.Bordet R, Ihl R, Korczyn AD, et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Med. 2017;15:107. doi: 10.1186/s12916-017-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderkova L, Rektorova I. Cognitive effects of repetitive transcranial magnetic stimulation in patients with neurodegenerative diseases — clinician’s perspective. J Neurol Sci. 2014;339:15–25. doi: 10.1016/j.jns.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Gomes-Osman J, Indahlastari A, Fried PJ, et al. Non-invasive brain stimulation: probing intracortical circuits and improving cognition in the aging brain. Front Aging Neurosci. 2018;10:177. doi: 10.3389/fnagi.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lett TA, Voineskos AN, Kennedy JL, et al. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol Psychiatry. 2014;75:361–70. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Wilcke T, Fuchs E, Funke K, et al. GABA — from inhibition to cognition: emerging concepts. Neuroscientist. 2018;24:501–15. doi: 10.1177/1073858417734530. [DOI] [PubMed] [Google Scholar]
- 35.Cirillo G, Di Pino G, Capone F, et al. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017;10:1–18. doi: 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2014;85:961–70. doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon JH, Grandelis A, Maddock RJ. Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J Neurosci. 2016;36:11788–94. doi: 10.1523/JNEUROSCI.1970-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169:302–11. doi: 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 39.Cheng CPW, Wong CSM, Lee KK, et al. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33:e1–13. doi: 10.1002/gps.4726. [DOI] [PubMed] [Google Scholar]
- 40.Lowe CJ, Manocchio F, Safati AB, et al. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: a systematic review and meta-analysis. Neuropsychologia. 2018;111:344–59. doi: 10.1016/j.neuropsychologia.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Kent PL. Working memory: a selective review. Appl Neuropsychol Child. 2016;5:163–72. doi: 10.1080/21622965.2016.1167491. [DOI] [PubMed] [Google Scholar]
- 42.Comijs HC, Kriegsman DM, Dik MG, et al. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Arch Gerontol Geriatr. 2009;48:191–6. doi: 10.1016/j.archger.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Andrews G, Halford GS, Shum DH, et al. Verbal learning and memory following stroke. Brain Inj. 2014;28:442–7. doi: 10.3109/02699052.2014.888758. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Li Z, Cong Y, et al. Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer’s disease patients. Oncotarget. 2017;8:33864. doi: 10.18632/oncotarget.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–72. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young-Bernier M, Kamil Y, Tremblay F, et al. Associations between a neurophysiological marker of central cholinergic activity and cognitive functions in young and older adults. Behav Brain Funct. 2012;8:17. doi: 10.1186/1744-9081-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang JH, Kim SH, Park CS, et al. Acute high-frequency rTMS of the left dorsolateral prefrontal cortex and attentional control in healthy young men. Brain Res. 2010;1329:152–8. doi: 10.1016/j.brainres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Vanderhasselt MA, De Raedt R, Baeken C, et al. A single session of rTMS over the left dorsolateral prefrontal cortex influences attentional control in depressed patients. World J Biol Psychiatry. 2009;10:34–42. doi: 10.1080/15622970701816514. [DOI] [PubMed] [Google Scholar]
- 49.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothkegel H, Sommer M, Rammsayer T, et al. Training effects outweigh effects of single-session conventional rTMS and theta burst stimulation in PD patients. Neurorehabil Neural Repair. 2009;23:373–81. doi: 10.1177/1545968308322842. [DOI] [PubMed] [Google Scholar]
- 51.Yamanaka K, Yamagata B, Tomioka H, et al. Transcranial magnetic stimulation of the parietal cortex facilitates spatial working memory: near-infrared spectroscopy study. Cereb Cortex. 2010;20:1037–45. doi: 10.1093/cercor/bhp163. [DOI] [PubMed] [Google Scholar]
- 52.Deppermann S, Vennewald N, Diemer J, et al. Does rTMS alter neurocognitive functioning in patients with panic disorder/agoraphobia? An fNIRS-based investigation of prefrontal activation during a cognitive task and its modulation via sham-controlled rTMS. Biomed Res Int. 2014;2014 doi: 10.1155/2014/542526. 542526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Jin J-N, Wang X, et al. Theta and alpha oscillations during the retention period of working memory by rTMS stimulating the parietal lobe. Front Behav Neurosci. 2017;11:170. doi: 10.3389/fnbeh.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wozniak-Kwasniewska A, Szekely D, Aussedat P, et al. Changes of oscillatory brain activity induced by repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in healthy subjects. Neuroimage. 2014;88:91–9. doi: 10.1016/j.neuroimage.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Jiménez-Balado J, Riba-Llena I, Abril O, et al. Cognitive impact of cerebral small vessel disease changes in patients with hypertension. Hypertension. 2019;73:342–9. doi: 10.1161/HYPERTENSIONAHA.118.12090. [DOI] [PubMed] [Google Scholar]
- 56.Bella R, Ferri R, Lanza G, et al. TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neurosci Lett. 2013;534:155–9. doi: 10.1016/j.neulet.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Abraham HMA, Wolfson L, Moscufo N, et al. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab. 2016;36:132–42. doi: 10.1038/jcbfm.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y-J, Tseng P, Chang C-F, et al. Modulating the interference effect on spatial working memory by applying transcranial direct current stimulation over the right dorsolateral prefrontal cortex. Brain Cogn. 2014;91:87–94. doi: 10.1016/j.bandc.2014.09.002. [DOI] [PubMed] [Google Scholar]