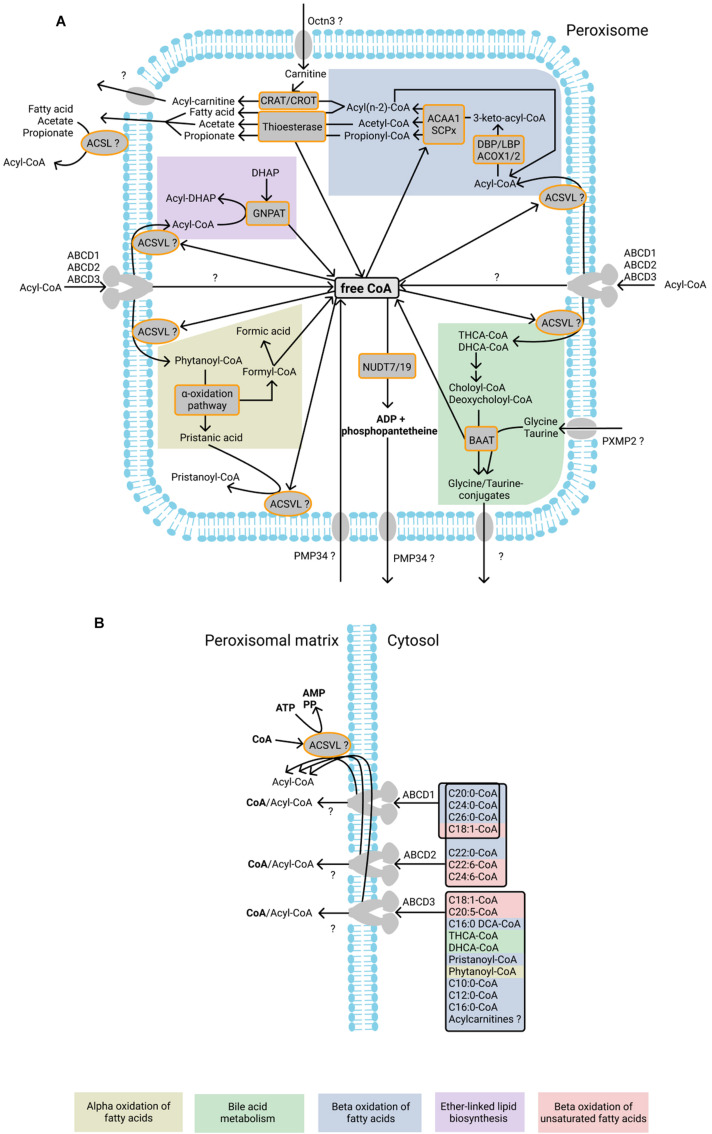
FIGURE 1.
Currently known CoA-dependent enzymatic reactions and transporters in human peroxisomes. (A) Fatty acids undergo beta-oxidation after import as acyl-CoA esters into peroxisomes. During beta-oxidation, acyl-CoAs are shortened to acyl(n-2)-CoA and acetyl-CoA molecules are produced. Peroxisomal beta-oxidation is mediated by the enzymes acyl-CoA oxidase 1, 2, and 3 (ACOX1, ACOX2, and ACOX3), L- and D-bifunctional protein (LBP and DBP), acetyl-CoA acyltransferase 1 (ACAA1), and sterol carrier protein x-related thiolase (SCPx). One CoA molecule is required for each circle of beta-oxidation. Acyl-CoA molecules produced during beta-oxidation may be hydrolyzed by thioesterases into fatty acids or acetate or converted to carnitine esters by CRAT and CROT, thereby producing free CoA. Free fatty acids and acetate can probably exit peroxisome directly after which ACSL, located on the cytosolic side of peroxisomal membrane, may be involved in the reactivation of the fatty acid and acetate. It is unclear which transporter is responsible for the export of acyl-carnitines. The peroxisomal enzyme GNPAT uses acyl-CoA esters for the acylation of DHAP during ether-linked lipid biosynthesis. During this reaction free CoA is released. Phytanoyl-CoA undergoes alpha-oxidation inside peroxisomes, resulting in the formation of formyl-CoA, which spontaneously splits into formic acid and CoA. Another product of alpha-oxidation – pristanic acid - is reactivated into pristanoyl-CoA by peroxisomal ACSVL. The bile acids THCA-CoA and DHCA-CoA are subjected to one cycle of beta-oxidation in peroxisomes during which choloyl-CoA and deoxycholoyl-CoA are produced and subsequently converted to glycine or taurine conjugates by BAAT. It is unknown how the conjugates are exported from the peroxisomes. It has been suggested that PMP34 imports free CoA into peroxisomes. The CoA diphosphohydrolases NUDT19 and NUDT7 degrade CoA into 3′,5′-ADP, and 4′-phosphopantetheine, which are subsequently exported from peroxisomes, possibly by PMP34. Proteins of the ABCD subfamily import acyl-CoA molecules into peroxisomes. During the import, the ester bond of acyl-CoA is hydrolyzed, and fatty acids subsequently undergo re-esterification by the intraperoxisomal ACSVL proteins. It is unknown whether the CoA molecule from acyl-CoA is also imported into the peroxisomal matrix after hydrolysis. (B) ABCD1, ABCD2, and ABCD3 transporters have different substrate affinities, as shown on the right. After the import of fatty acids, they are esterified by the ACSVL proteins in a CoA- and ATP-dependent reaction. ABCD proteins were shown to hydrolyze the ester bond in the CoA esters during import, although it has also been suggested that ABCD transporters import acyl-CoA molecules without hydrolysis of the ester bond (see text for more information). Enzymatic reactions or molecules belonging to the same metabolic pathway are marked with background color and listed at the bottom.