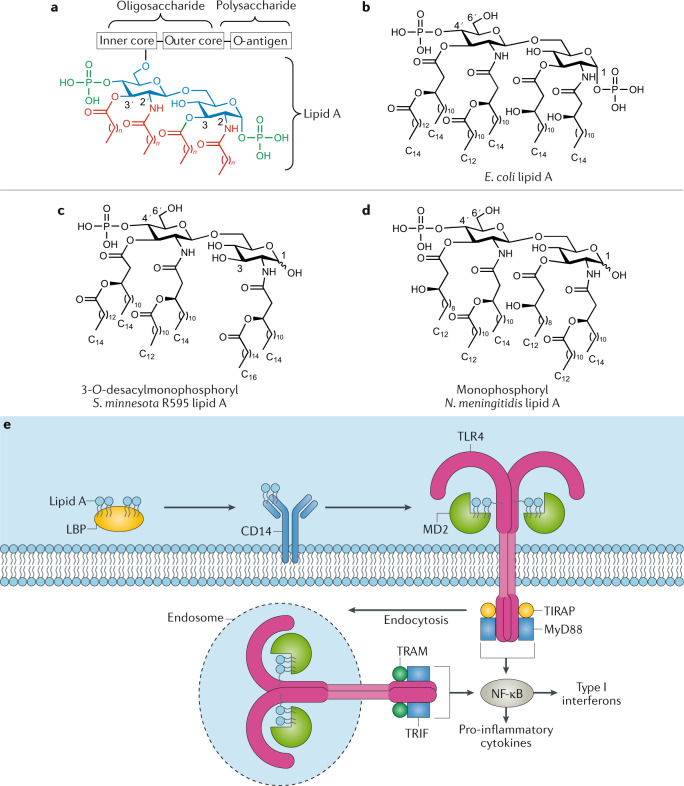
Fig. 3. Structures of lipid A and monophosphoryl lipid A and its mechanism of action.
a | General structure of lipopolysaccharide (LPS) with its three main domains, lipid A, core oligosaccharide and O-antigen polysaccharide. b | Structure of natural lipid A from Escherichia coli134. c,d | Structures of chemically modified monophosphoryl lipid A from Salmonella minnesota R595 (clinically approved, panel c)147 and from Neisseria meningitidis (panel d)134. e | Schematic representation of LPS/Toll-like receptor 4 (TLR4) signalling pathway and lipid A mechanism of action166. LPS-binding protein (LBP)-associated LPS (or lipid A) is first transferred to CD14 and then delivered to myeloid differentiation factor 2 (MD2), enabling formation of the LPS–MD2–TLR4 ternary complex, which drives dimerization of TLR4 receptors. LPS/TLR4 signalling involves intracellular recruitment of Toll/interlukin-1 (IL-1) receptor domain-containing adapter protein (TIRAP), TRIF-related adapter molecule (TRAM), myeloid differentiation factor 88 (MyD88) and Toll/IL-1 receptor domain-containing adapter inducing interferon-β (TRIF) co-receptors. While the MyD88-dependent pathway promotes pro-inflammatory cytokine expression, the MyD88-independent pathway mediates the induction of type I interferons. Part e adapted with permission from ref.132, Wiley, and with permission from ref.166, Elsevier.