Scheme 1. Mechanistic Study.
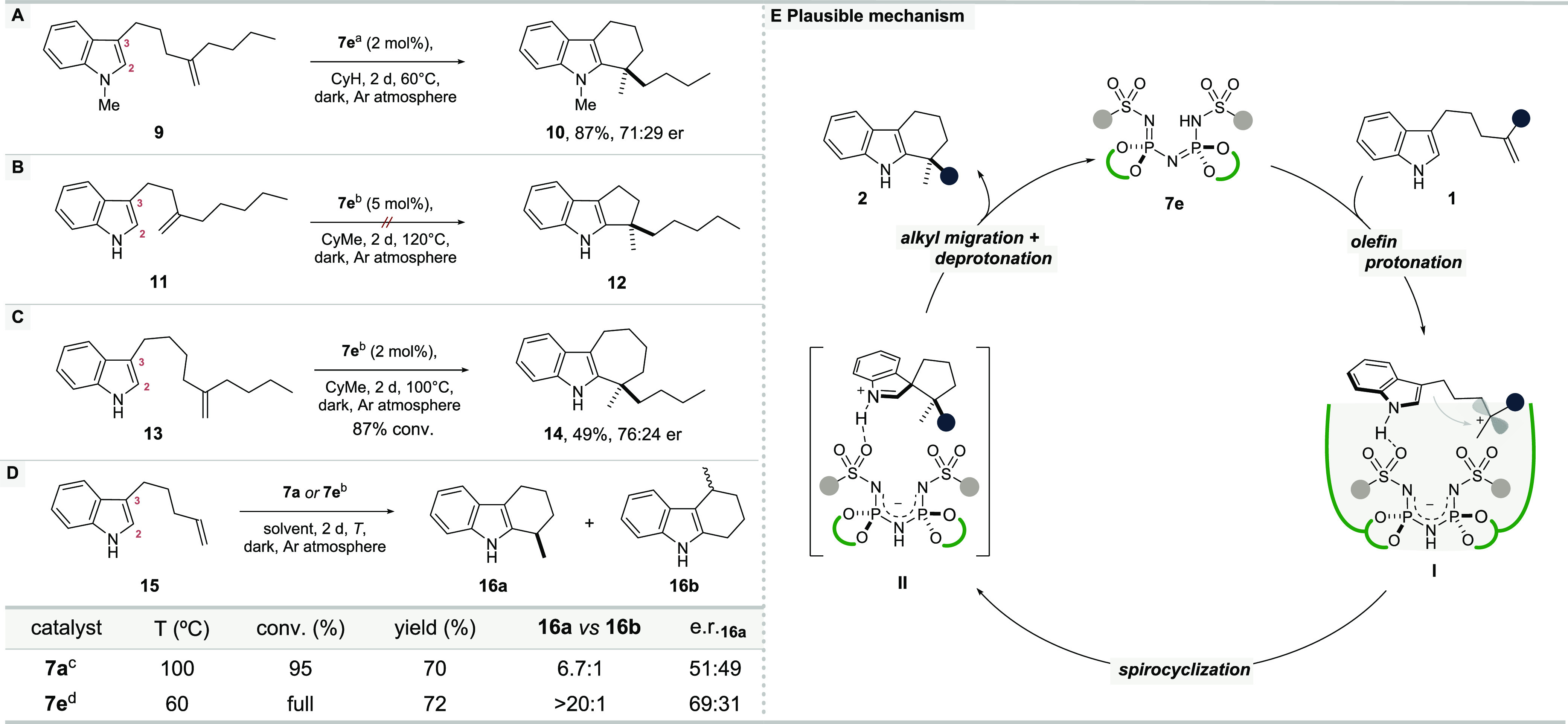
Reaction was carried out with 0.2 mmol of 9, catalyst 7e (2 mol %) in cyclohexane (0.1 M); yield is of for the isolated compound; enantiomeric ratios (er) were measured by HPLC.
Reactions were performed with substrate 11, 13, 15 (0.02 mmol); conversions, yields, and regioisomeric ratios were determined by 1H NMR analysis with mesitylene as an internal standard.
100 °C with 4 mol % 7a in methylcyclohexane; 60 °C with 2 mol % 7a in cyclohexane, trace product.
60 °C with 2 mol % 7e in cyclohexane.
