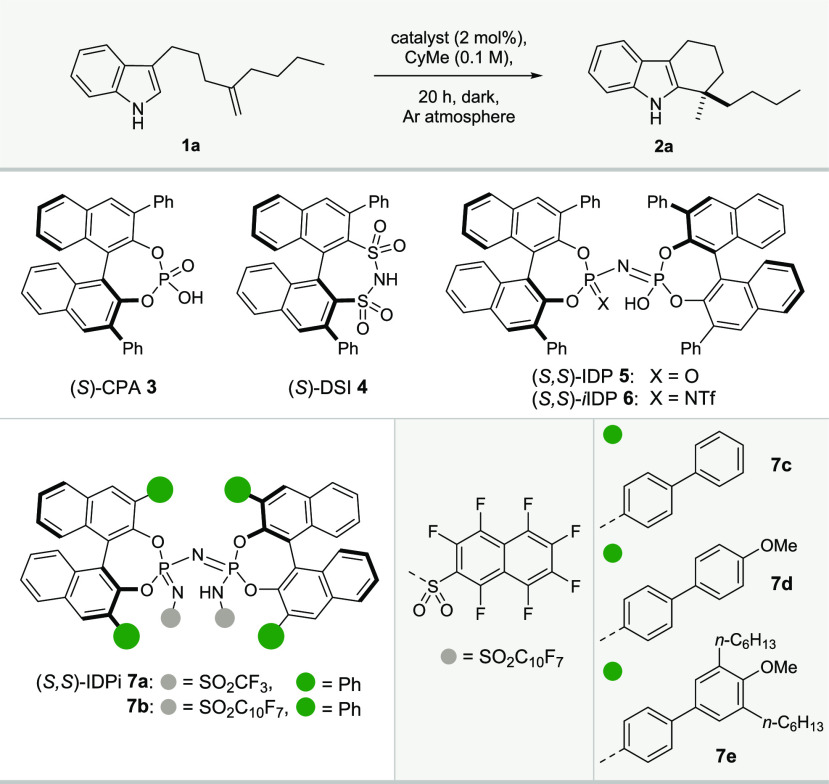
Table 1. Reaction Developmenta.
| entry | catalyst | T (°C) | conv. (%) | yield 2a (%) | isom. (%) | er |
|---|---|---|---|---|---|---|
| 1 | 3 | 80 | 41 | 34 | 5 | 54:46 |
| 2 | 4 | 80 | 47 | 30 | trace | 44:56 |
| 3 | 5 | 80 | 13 | <10 | trace | 53:47 |
| 4 | 6 | 80 | 55 | 45 | 9 | 47:53 |
| 5 | 7a | 80 | 95 | 90 | trace | 49:51 |
| 6 | 7b | 80 | full | 93 | — | 56:44 |
| 7 | 7c | 80 | full | 85 | — | 88:12 |
| 8 | 7d | 80 | full | 93 | — | 88:12 |
| 9 | 7e | 80 | full | 93 | — | 90:10 |
| 10b | 7e | 60 | full | 95 | — | 95:5 |
Reactions were performed with substrate 1a (0.02 mmol), catalyst (2 mol %) in methylcyclohexane (CyMe, 0.2 mL); conversions (conv.), yields of 2a, and olefin isomerizations (isom.) were determined by 1H NMR analysis with mesitylene as an internal standard; enantiomeric ratios (er’s) were measured by HPLC. When the reaction was not carried out in the dark, byproducts and lower yields were observed.
48 h.