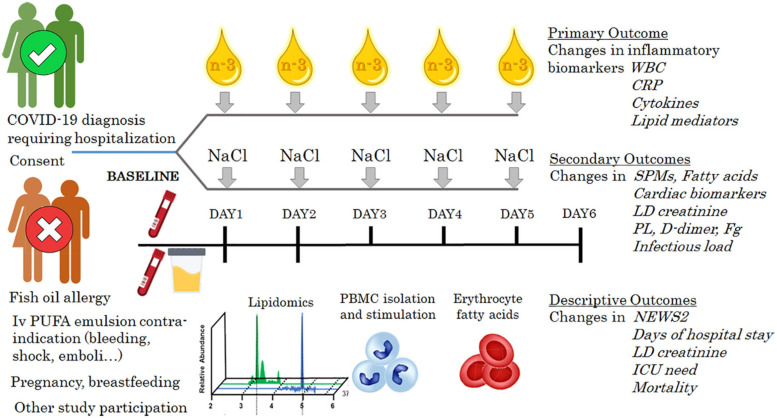
FIGURE 2.
Schematic study protocol for the COVID-Omega-F trial, registered in the European Union Drug Regulating Authorities Clinical Trials (EudraCT) database with number 2020-002293-28 and at ClinicalTrials.gov (clinicaltrials.gov/ct2/show/NCT04647604). An intravenous omega-3 PUFA emulsion (n-3) is compared with placebo (NaCl) for effects on a panel of inflammatory biomarkers; white blood cells (WBC), C-reactive protein (CRP), cytokines and lipid mediators at the end of the treatment period. Secondary endpoints include changes in specialized proresolving mediators (SPMs), fatty acids, cardiac markers, lactate dehydrogenase (LD), D-dimer, fribronogen (Fg), and infectious load. National Early Warning Score 2 (NEWS2), length of hospital stay, intensive cae unit (ICU) need, and mortality will be monitored for a descriptive analysis. The inclusion criteria for COVID-Omega-F comprise female and male patients ≥18 years of age diagnosed with COVID-19 and presenting with a clinical status requiring hospitalization. Exclusion criteria are defined according to i.v. omega-3 PUFA emulsion contraindications, known hypersensitivity, participation in other clinical research study, pregnancy, and breastfeeding.