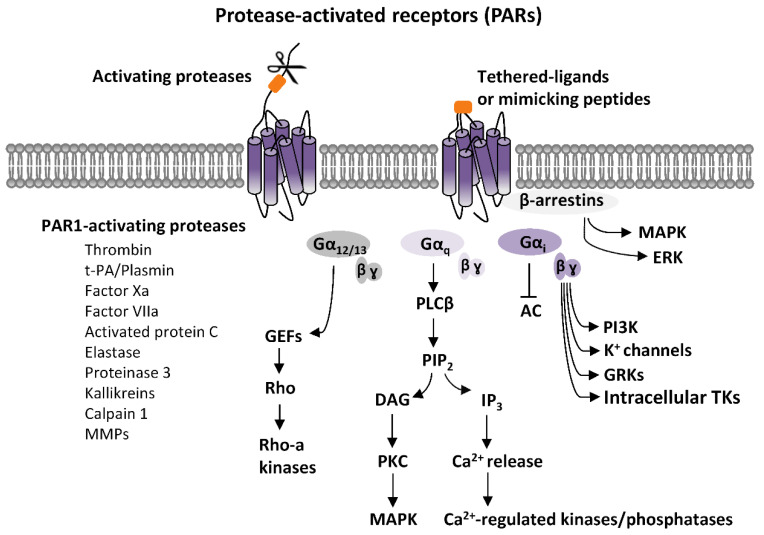
Figure 1.
Protease-activated receptors (PARs) signaling. Scheme of the principal PARs-dependent signaling pathways. PARs activation, elicited by proteases-induced unmasking of tethered ligands or, alternatively, by ligand-mimicking peptides, stimulates various G proteins-dependent pathways. Gαq-mediated activation of phospholipase C β (PLCβ) results in the hydrolysis of phosphatidylinositol and generation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), that fosters Ca2+ intracellular mobilization from internal stores, and activation of Ca2+-regulated kinases and phosphatase. Protein kinase C (PKC), which is also activated by DAG, stimulates mitogen-activated protein kinases (MAPK). PARs coupling with Gα12/13, that binds to guanine nucleotide exchange factors (GEFs), results in the activation of the small soluble G protein, Rho, and, consequently, of Rho-activated kinases. PARs-induced activation of Gi, inhibits, via Gαi,, adenylyl cyclase (AC), and, via the βγ subunit, produces opening of G protein-activated inward rectifying K+ channels (GIRK), the activation of G protein-coupled receptor kinases (GRKs), as well as the stimulation of intracellular tyrosine kinases (TKs), and the activation of phosphotydil-inositole-3-kinase (PI3K), that then activates other kinases signaling pathways, including mitogen-activated protein kinase (MAPK). Additionally, PARs stimulations can activate G protein-independent mechanisms, mediated by the recruitment of β-arrestins and activation of diverse signaling pathways, including MAPK-like extracellular signal-regulated kinases (ERK).