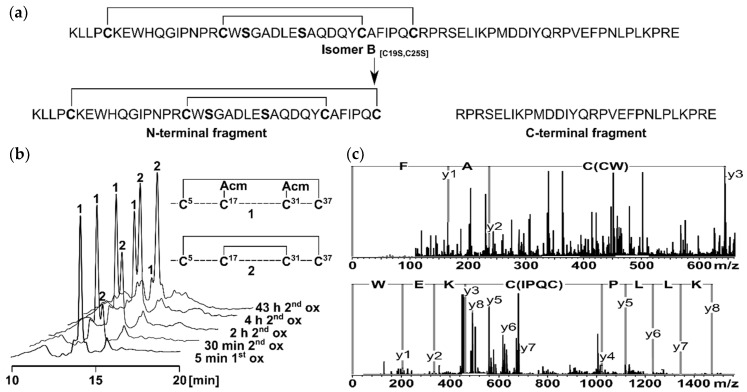
Figure 1.
(a) Derivation of the two fragments from the two-disulfide-bonded tridegin isomer B[C19S,C25S] [29]. The N-terminal fragment consists of amino acid 1–37 and contains the disulfide bonds Cys5–Cys37 and Cys17–Cys31. The C-terminal fragment is composed of amino acids 38–66 without a cysteine residue; (b) HPLC elution profiles of the stepwise oxidation strategy applied for the synthesis of the N-terminal fragment. During the first oxidation (1.1 eq. iodine, 100% AcOH) the first disulfide bond between Cys5 and Cys37 was formed (1). Subsequently the second disulfide bond between Cys17 and Cys31 was built (2) by increasing the amount of iodine (15 eq.) and adjusting the concentration of AcOH (70%); (c) MS/MS analysis of the N-terminal fragment after digestion with chymotrypsin. The disulfide bond connectivity was confirmed by the characteristic MS/MS fragments C(CW) AF and KLLP C(IPQC) KEW.