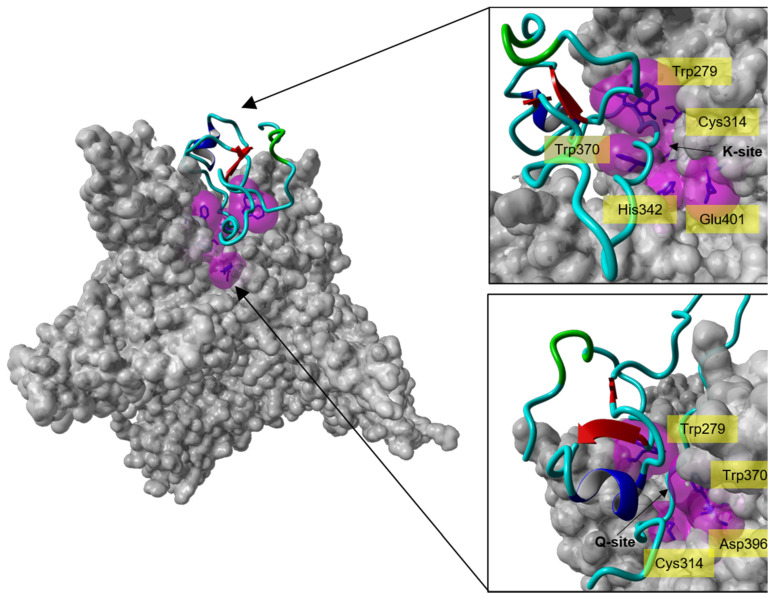
Figure 6.
Molecular docking analysis of the interaction of isomer B[C19S,C25S] [29] with the catalytic site of FXIIIa. Left: Crystal structure of the calcium-activated FXIIIa-monomer (PDB ID: 4KTY [16], grey surface) docked with one of the top-ten-ranked conformations of isomer B[C19S,C25S] [29] (cyan ribbon), which interacts with the catalytic site of FXIIIa. Right: Zoom-in from different directions (lysine access site (top, K-site) and glutamine access site (bottom, Q-site)) of the FXIIIa active site interacting with isomer B[C19S,C25S] [29]. The active site residues of FXIIIa (Cys314, His373, Asp396, Trp279, and Trp370) are shown as blue sticks enclosed by their molecular surfaces (violet translucent surface).