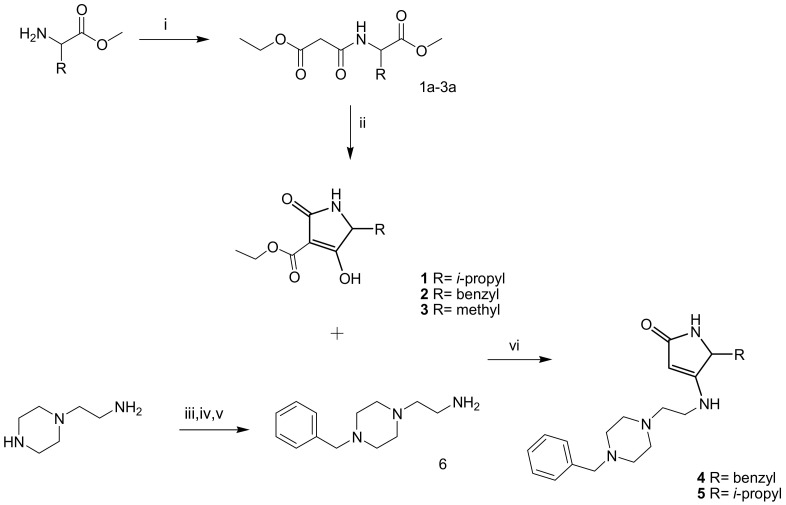
Scheme 1.
Reagents and conditions: (i) ethyl malonyl chloride, triethylamine, anhydrous CH2Cl2, N2, 0 °C, 24 h; (ii) Na, abs. ethanol, reflux 4 h; (iii) phthalic anhydride, 160 °C, 4 h; (iv) benzyl bromide, KOH, 96° ethanol, 24 h RT; (v) aq solution of MeNH2 (40% w/w), 60 h, RT; (vi) 1 or 2, THF, 5 h, reflux.