Table 2.
Stepwise protonation constant of compound 1 and global formation constants a of its Fe(III), Cu(II) and Zn(II) complexes (T = 25.0 ± 0.1 °C, I = 0.1 M KCl, 15% w/w DMSO/water) and pM b values.
| Compound | MmHhLl (m,h,l) |
log K | log β(FemHhLl) | log β(CumHhLl) | log β(ZnmHhLl) |
|---|---|---|---|---|---|
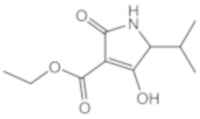 1 |
(011) | 2.334(5) c | |||
| 2.85(4) d | |||||
| (101) | 9.46(7) d | 4.08(8) d | 3.16(8) c | ||
| (1-21) | - | −4.15(7) d | −11.75(5) c | ||
| (102) | 14.30(7) d | - | - | ||
| (1-12) | - | 6.56(6) d | - | ||
| (1-22) | 5.83(8) d | - | - | ||
| (103) | 19.4(1) d | - | - | ||
| pM | 16.6 | 11.6 | 6.0 |
a βMmHhL1 = [MmHhLl]/[M]m[H]h[L]l; b pM = −log[M] at pH 7.4 (CL/CM = 10, CM = 10−6 M) [38]; c potentiometric values; d spectrophotometric values.
