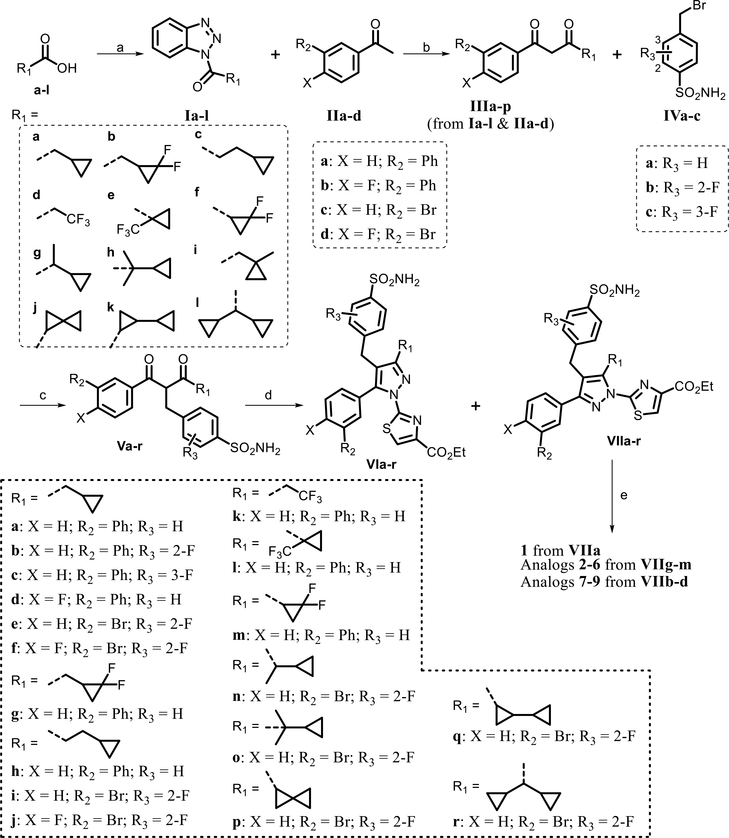
Scheme 1.
Syntheses of Intermediates VIa–r and Analogues 1–9a
aReagents and conditions: (a) SOCl2, CH2Cl2, 4 h, and 91–100%; (b) MgBr2·OEt2, iPr2NEt, CH2Cl2, 12 h, and 60–69%; (c) Cs2CO3, DMSO, 1 h, and 55–83%; (d) (i) pyrrolidine (0.5 equiv), TsOH (0.5 equiv), EtOH, reflux, and 1–2 h and (ii) ethyl 2-hydrazinylthiazole-4-carboxylate·HBr and reflux overnight; and (e) LiOH, THF/MeOH/H2O, and 1 h.