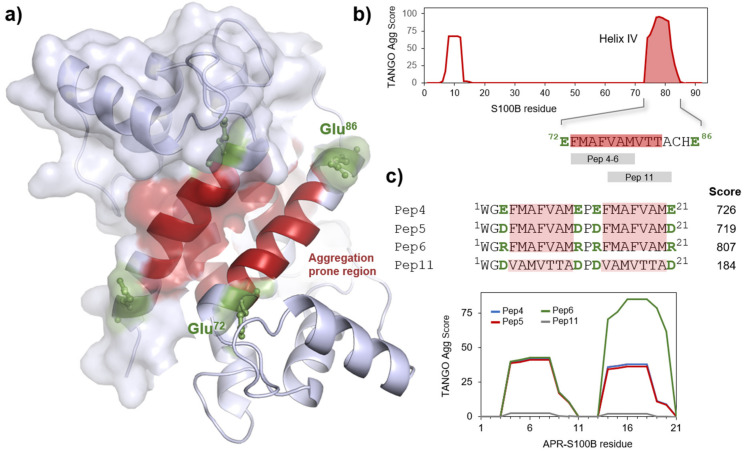
Figure 1.
S100B structure and designed aggregation-prone peptides. (a) Structure of the human S100B dimer with the aggregation-prone helix IV marked in red and its capping Glu gatekeeper residues depicted in green; one of the subunits is marked with surface contour. Figure made with Pymol [25] based on the Protein Data Bank (PDB) entry 3d0y; (b) Plot of TANGO scores of human S100B, highlighting helix IV and its APR sequence; (c) Sequence of the designed APR-S100B peptides with the tandem APR highlighted in red, gatekeepers in bold green and plots of TANGO scores.