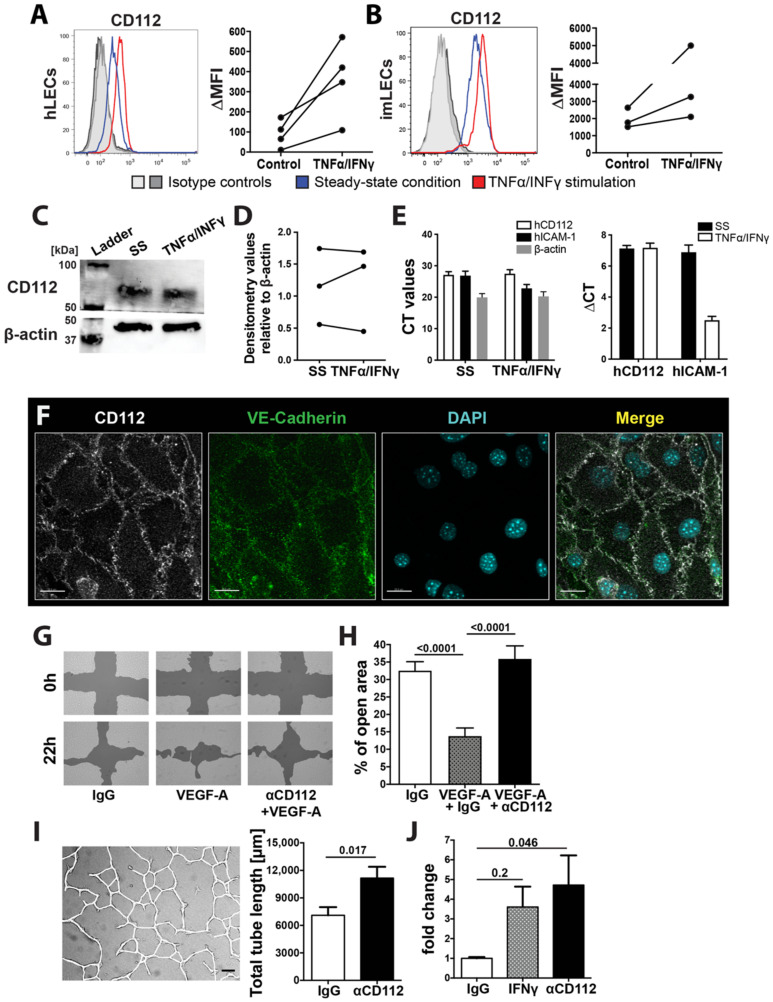
Figure 2.
CD112 regulates endothelial cell function in vitro. (A,B) Flow cytometry analysis showing CD112 expression by human (A) and conditionally immortalized murine LECs (imLECs) (B). Representative flow cytometry plots of CD112 expression comparing steady-state (blue line) and inflamed conditions (red line: TNFα/IFNγ treated; grey lines: isotype controls) are shown on the left. Summary of MFI values of CD112 expression of three to four experiments are shown on the right. Data points of the same experiment are connected by a line. (C) Western blot analysis configure 112. protein (~65 kDa) expression in in vitro cultured human LECs in steady-state (SS) and upon TNFα/IFNγ-mediated inflammation. β-actin was used as an internal control. Blots were cut at the 50 kDa ladder mark. Representative data from one out of three experiments are shown. (D) Summary of densitometry signal intensities of CD112 protein in steady state (SS) and upon TNFα/IFNγ-mediated inflammation normalized to β-actin signal of three Western blot experiments. Data points from the same experiment are connected by a line. (E) Quantitative real-time PCR analysis of CD112 mRNA levels in in vitro cultured human LECs in steady-state (SS) and upon TNFα/IFNγ-mediated inflammation. Induction of ICAM-1 mRNA levels by TNFα/IFNγ was analysed as a positive control. Delta cycle threshold (CT)values: Difference between the CT values measured for CD112 and for the housekeeping gene RPLP0. Pooled data (experimental means) from three independent experiments (biological replicates) are shown. Each experiment was performed in triplicate (technical replicates). (F) Representative immunofluorescence images of CD112 expression (white) on in vitro cultured imLEC monolayers colocalizing with the junctional molecule VE-cadherin (green) at the intercellular junctions. Scale bar: 20 μm. (G,H) Human LEC migration upon CD112 blockade was investigated in an in vitro scratch assay. (G) Representative images of an LEC scratch assay in VEGF-A-induced wound closure. Scale bars: 200 µm. (H) Pooled quantitative analysis from three independent experiments are shown. (I) Representative image (left) of an in vitro tube formation assay. Confluent human LEC monolayers were incubated overnight in collagen gel solution supplemented with blocking antibody for CD112 or isotype control. Total tube length was manually analysed using a self-made macro in FIJI (ImageJ). Quantitative analysis (right) of total tube length upon CD112 blockade. One out of three similar independent experiment is shown. (J) In vitro permeability assay, in which 70 kDa FITC dextran was applied onto confluent pretreated LEC monolayers grown on a Boyden transwell membrane. After 30 min, diffused FITC dextran was quantified in the lower chamber. Pooled data from three similar and independent experiments are shown.