Figure 1: Experimental workflow.
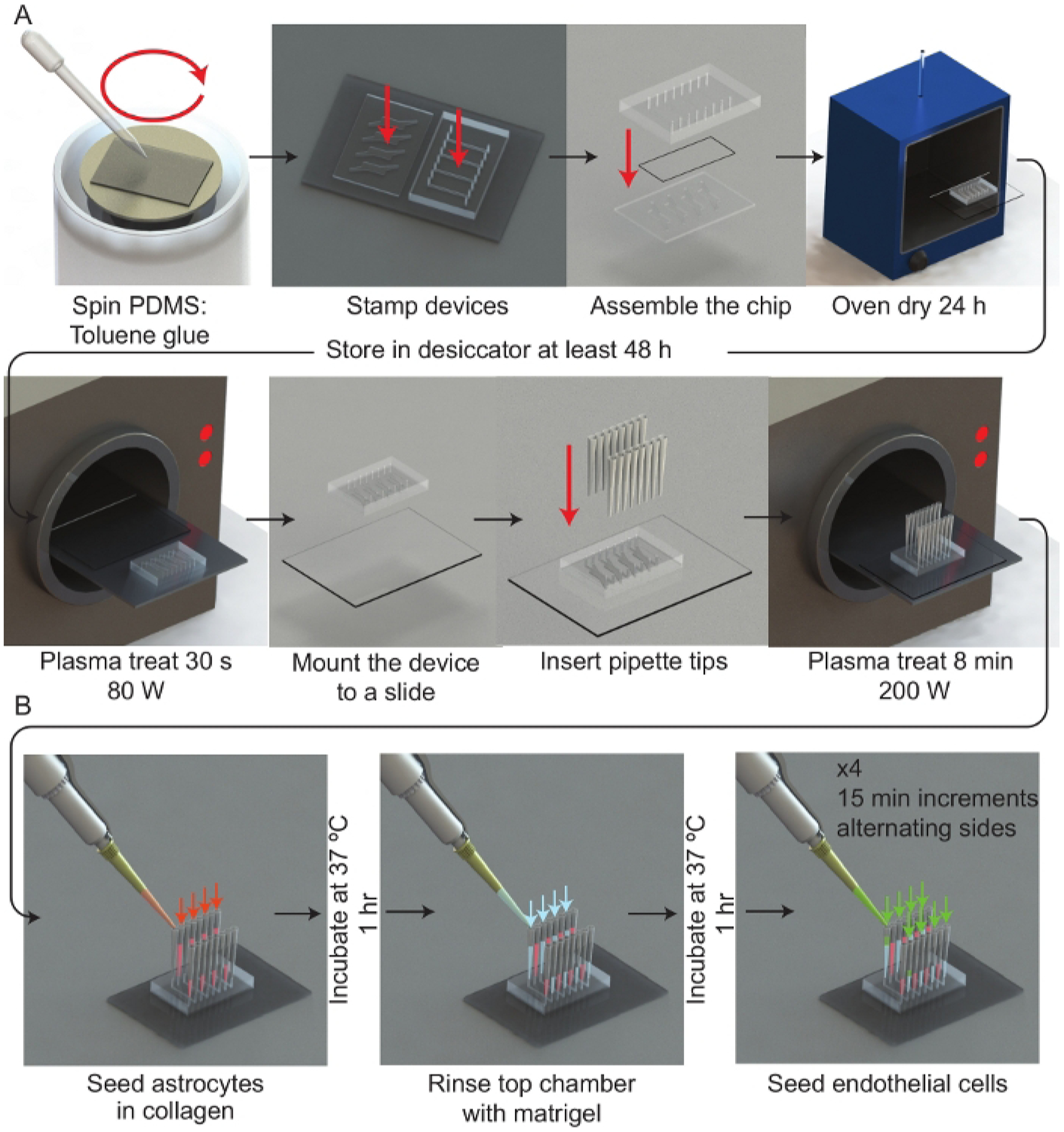
(A) Schematic representation of the microfluidic device assembly process. A spinner is used to deposit a thin film of PDMS:toluene glue onto a 50 mm × 75 mm glass slide. Each half of the μmBBN device is stamped channel side facing the glue and then assembled with a polycarbonate membrane (5 μm pores) between the μmBBN device parts. The μmBBN devices are placed in a 37 °C oven for 24 h to cure the glue. Devices are then dried in a vacuum desiccator for at least 48 h prior to experimental use. A μmBBN device and 50 mm × 75 mm glass slide are activated with a plasma treatment and bonded together. Standard P200 pipettes cut at the tips are inserted into all μmBBN device inlets and outlets. The completed μmBBN device is then sterilized by an 8 min (200 W) plasma treatment then transferred to a sterile secondary container. (B) Schematic overview of utilizing the microfluidic device scaffolding to create a cellular blood brain barrier and brain niche micro-environment. Inside a biosafety cabinet, a mixture of astrocytes in collagen are seeded into the bottom μmBBN device chamber and allowed to solidify for 1 h at 37 °C. Matrigel is used to coat the membrane through the top flow chamber for 1 h at 37 °C. Then endothelial cells are seeded into one tip of the top chamber and allowed to flow and settle for 15 min. This seeding is repeated x4, alternating sides of the flow chamber.
