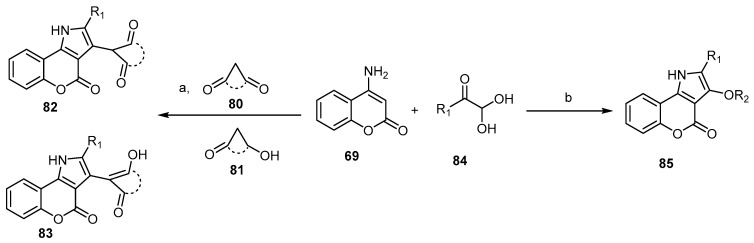
Scheme 24.
Various arylglyoxals in the synthesis of [l]benzopyrano[4,3-b]pyrrol-4-ones 82, 83, and 85. Reagents and conditions: (a) i: AcOH, reflux, 40 min; ii: an appropriate alkyl p-toluenesulfonate (TsOR2), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), toluene, reflux, 1.5 h, 14 outputs with 73%–89% yield; (b) R1=Ph, 4-CH3OC6H4, 4-CH3C6H4, 4-FC6H4, 4-ClC6H4, 4-BrC6H4, 2-thienyl; dimedone, 2-hydroxy-1,4-naphtoquinone barbituric acid, 1,3 dimethyl barbituric acid, I2, DMSO, stirring, 100 °C, 7 h, 15 outputs with 15%–80% yield.