Abstract
Background
In liver transplant (LT) recipients with severe coronavirus disease 2019 (COVID-19), fatal outcome has been reported in a substantial subset of patients. Whether LT recipients are at increased risk for severe COVID-19 compared with the general population is controversial. Here we report the results of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serosurvey in a large LT recipient cohort.
Methods
A total of 219 LT recipients were enrolled between May 5, 2020, and August 6, 2020, at the University Hospital Heidelberg. Serum blood samples were collected and tested for anti–SARS-CoV-2 IgG. SARS-CoV-2 RNA was detected in nasopharyngeal swabs using reverse transcription–polymerase chain reaction assays.
Results
Taking into account known risk factors of arterial hypertension, obesity, diabetes, or leukopenia, LT recipients a priori represented a high-risk cohort for severe COVID-19 with 101 of 219 (46.1%) presenting with more than 2 risk factors for severe COVID-19. Out of 219 LT recipients, 8 (3.7%) either had a positive test result for nasopharyngeal SARS-CoV-2 RNA or anti–SARS-CoV-2 serum IgG. Five of eight (62.5%) did not show any clinical signs of infection, three of eight (37.5%) had self-limited disease, and none required hospitalization for COVID-19. Two of eight (25%) had known exposure to infected health care staff as the probable source of infection.
Conclusions
In summary, LT recipients showed a SARS-CoV-2 seroconversion rate similar to that of the general population with a substantial percentage of unrecognized infections.
Liver transplant (LT) recipients are a patient group vulnerable to severe infections due to immunosuppression. Early published case series in LT and solid organ transplant (SOT) recipients with coronavirus disease 2019 (COVID-19) reported a high fatality rate of up to 30%, exceeding the rate of the general population [[1], [2], [3], [4]]. In contrast, a more recent Swiss case study and an international registry study found mortality rates comparable to those of comorbidity-matched non–transplant recipients [5,6]. Initial symptoms of patients with COVID-19 are diverse [7]. Patients who have a positive test result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be asymptomatic or can develop COVID-19, and in severe cases, their condition may deteriorate due to acute respiratory distress syndrome with subsequent respiratory failure and death [8]. Both among the general population and among SOT recipients, the main risk factors for severe COVID-19 were older age and comorbidities such as diabetes, obesity, and renal and cardiopulmonary disease [[9], [10], [11], [12]]. Data on immunosuppression as a risk factor for COVID-19 are inconclusive, but minimization of immunosuppression has been widely recommended for LT patients with COVID-19 on the basis of experience with other respiratory pathogens [13]. LT patients in most centers are therefore counseled to strictly follow hygiene and lifestyle precautions to avoid SARS-CoV-2 infection [14]. It is not known to what extent this advice is effective to reduce SARS-CoV-2 infection in the LT recipient population. The present study aimed to define the incidence and prevalence of SARS-CoV-2 infection and to explore the (preventive) effect of personal behavioral adjustments among LT recipients.
Methods
Study Design
This prospective study was conducted between May 5, 2020, and August 6, 2020, at the University Hospital Heidelberg, located in the German state of Baden-Württemberg. All study participants had undergone LT in the past and were older than 18 years of age. All study participants provided written informed consent before enrollment. The study was approved by the ethics committee of the Medical Faculty of the University of Heidelberg (approval number S-457/2020). The study was conducted in accordance with good clinical practice principles and the Declaration of Helsinki.
Sampling and Procedures
Study enrollment was performed when patients presented for scheduled routine follow-up after LT. Routine follow-up on an outpatient basis is performed at least every 6 months at our center. After patients provided written informed consent, their serum blood samples were collected between May 5 and August 6, 2020. Blood was centrifuged, and serum was stored until analysis (−80°C). Analyses were performed in batches at the University Hospital Heidelberg. Anti–SARS-CoV-2 IgG (EUROIMMUN) was determined with enzyme-linked immunosorbent assays (ELISAs). The manufacturer's data sheet (April 29, 2020) reports cross-reactivities with anti–SARS-CoV-1 IgG antibodies, but not with Middle East respiratory syndrome coronavirus, human coronavirus (HCoV)-229E, HCoV-NL63, HCoV-HKU1, or HCoV-OC43 IgG antibodies. Manufacturer-reported sensitivity and specificity were 94.6% and 99.8%, respectively. Routine pharyngeal and nasal SARS-CoV-2 swabbing was performed on 126 patients between May 5, 2020, and July 5, 2020. Thereafter, routine SARS-CoV-2 screening was terminated because general population infection rates were very low. Nasopharyngeal swabs underwent nucleic acid amplification testing (reverse transcription–polymerase chain reaction [RT-PCR]) on the same day to confirm SARS-CoV-2 infection in certified test laboratories at University Hospital Heidelberg. For diagnosis of SARS-CoV-2, RNA was isolated from nasopharyngeal and oropharyngeal swab specimens using QIAGEN kits (QIAGEN, Hilden, Germany), automated on the QIASymphony (DSP Virus/Pathogen Mini Kits) or QIAcube (QIAamp Viral RNA Mini Kits) devices, and eluted in 115 μL of elution buffer. RT-PCR was carried out using various reagent mixes according to the manufacturers’ instructions: LightMix Modular SARS and Wuhan CoV E-gene, LightMix Modular SARS and Wuhan CoV N-gene, LightMix Modular Wuhan CoV RdRP-gene, and LightMix Modular EAV RNA Extraction Control (as an internal control) from TIB MOLBIOL Syntheselabor GmbH (Berlin, Germany) and the LightCycler Multiplex RNA Virus Master (Roche, Mannheim, Germany). RT-PCR was performed on LightCycler 480 or 480 II (Roche). Additionally, 104 patients answered a questionnaire to assess behavior changes due to the COVID pandemic.
Statistics
Statistical analysis was carried out by a statistician using Prism (version 4.4.1; GraphPad Software) or IBM SPSS Statistics (version 22) software as appropriate. P values are given as indicated using the χ2 test or a nonparametric t test.
Results
We conducted a prospective study of 219 LT recipients at an LT center in the south of Germany, with sample collection taking place between May 5 and August 6, 2020. LT patients are a highly comorbid patient group. In our study, only 55 of 219 (25.1%) had no known risk factor for a severe course of COVID-19 (diabetes, hypertension, obesity, cardiac disease, leukopenia), whereas 63 of 219 (28.8%) had one risk factor and 101 of 219 (46.1%) had two or more risk factors. Particularly, arterial hypertension (108 of 219 [49.3%]) and type 2 diabetes mellitus (66 of 219 [30.1%]) were highly prevalent among our study participants (Table 1 ). Routine pharyngeal and nasal SARS-CoV-2 swabbing was performed on 126 patients between May 5, 2020, and July 5, 2020. Thereafter, routine SARS-CoV-2 screening was terminated because general population infection rates were very low. Two of 126 (1.5%) patients had positive test results for nasopharyngeal SARS-CoV-2 RNA (Table 2 ). Both SARS-CoV-2 RNA-positive patients (patients 1 and 2; Table 3 ) presented for planned routine LT follow-up meetings but reported symptoms of uncomplicated upper respiratory tract infection.
Table 1.
Clinical Characteristics
| Characteristics | All Patients (N = 219) | SARS-CoV-2–Positive Patients (n = 8) | P Value |
|---|---|---|---|
| Sex, female, n (%) | 89 (40.6%) | ns | |
| Age, years (median, range) | 56.9 (18.1-78.2) | 56.7 (25.5-69.5) | ns |
| >65 years | 44 (20.8%) | 2 (25.0%) | ns |
| Interval from LT, years | 6.4 (0.1-30.0) | 6.5 (0.2-15.0) | ns |
| Indication for LT | |||
| Alcohol | 42 (19.2%) | 0 (0.0%) | ns |
| Hepatitis B | 9 (4.1%) | 1 (12.5%) | ns |
| Hepatitis C | 10 (4.6%) | 0 (0.0%) | ns |
| HCC | 43 (19.6%) | 2 (25.0%) | ns |
| PSC | 26 (11.9%) | 0 (0.0%) | ns |
| Others | 79 (36%) | 5 (62.5%) | ns |
| Comorbidities | |||
| BMI, kg/m2 (mean, SD) | 25.6 (15.8-46.0) | 22.9 (20.0-32.2) | ns |
| <25 | 97 (44.3%) | 5 (62.5%) | |
| 25-30 | 74 (33.8%) | 1 (12.5%) | |
| >30 | 48 (21.9%) | 2 (25%) | |
| Cardiovascular disease | 31 (14.2%) | 1 (12.5%) | ns |
| Respiratory disease | 21 (9.6%) | 0 (0.0%) | ns |
| Hypertension | 108 (49.3%) | 5 (62.5%) | ns |
| Diabetes | 66 (30.1%) | 2 (25.0%) | ns |
| Active cancer | 3 (1.4%) | 0 (0.0%) | ns |
| Active smoking | 19 (8.7%) | 0 (0.0%) | ns |
| Leukopenia | 50 (22.8%) | 2 (25%) | ns |
| RAAS inhibitor use | 56 (25.6%) | 1 (12.5) | ns |
| Renal insufficiency | |||
| Dialysis | 11 (5.0%) | 2 (25%) | .02 |
| GFR <30 mL/min | 17 (7.7%) | 2 (25%) | |
| GFR 30-60 mL/min | 43 (19.6%) | 2 (25%) | |
| GFR <60 mL/min | 159 (72.6%) | 4 (50%) | |
| Immunosuppression | |||
| Tacrolimus | 140 (63.9%) | 5 (62.5%) | ns |
| Cyclosporine | 42 (19.2%) | 2 (25%) | ns |
| mTOR inhibitor | 54 (24.7%) | 2 (25%) | ns |
| MMF/MPA | 97 (44.3%) | 4 (50%) | ns |
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; HCC, hepatocellular carcinoma; LT, liver transplant; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; PSC, primary sclerosing cholangitis; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Table 2.
SARS-CoV-2 Test Results
| Anti-SARS-CoV-2 IgG Negative | Anti-SARS-CoV-2 IgG Positive | Total | |
|---|---|---|---|
| SARS-CoV-2 RNA Negative | 211 (96.3%) | 6 (2.7%) | 217 (99.0%) |
| SARS CoV-2 RNA Positive | 1 (0.5%) | 1 (0.5%) | 2 (1.0%) |
| Total | 212 (96.8%) | 7 (3.2%) |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Clinical Patient Details
| Patient No. | Sex | RNA | IgG | Age (Years) | Time Since LT (Years) | Clinical Symptoms | Dialysis | Hypertension | Diabetes | BMI (kg/m²) | Immunosuppression |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | + | + | 25 | 0.5 | Fever, dry cough, fatigue | No | No | No | 23 | Tac/MMF |
| 2 | F | + | − | 51 | 6.8 | Dry cough | Yes | Yes | No | 30 | Cic |
| 3 | M | − | + | 62 | 0.2 | Dry cough, fever | No | Yes | Yes | 30 | Tac/MMF |
| 4 | M | − | + | 60 | 3.6 | None | No | Yes | Yes | 32 | Tac/mTOR |
| 5 | F | − | + | 51 | 6.2 | None | No | Yes | No | 22 | Tac/MMF |
| 6 | M | − | + | 67 | 7.4 | None | No | No | No | 20 | Tac/MMF |
| 7 | M | − | + | 52 | 14.6 | None | No | No | No | 23 | Tac |
| 8 | F | − | + | 69 | 15.0 | None | Yes | Yes | No | 21 | mTOR |
Abbreviations: BMI, body mass index; Cic, ciclosporin; LT, liver transplant; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; Tac, tacrolimus.
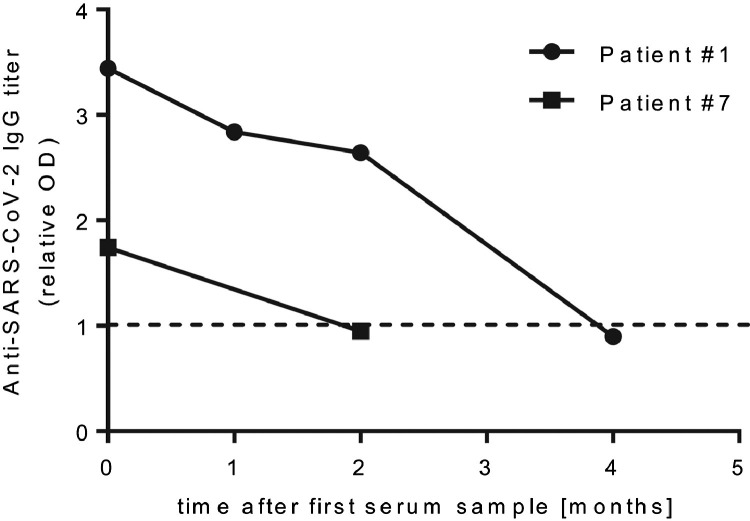
We further collected 241 serum samples from 219 LT recipients presenting for routine follow-up between May 5 and August 6, 2020, in southern Germany (Fig 1 ). We performed a semiquantitative ELISA on anti–SARS-CoV-2 IgG directed against its spike protein. Of these 241 samples, 13 (5.4%) tested positive for anti–SARS-CoV-2 IgG, representing 7 individual patients. Some patients had repeat serum samples, and all of them were concordantly positive or negative for anti–SARS-CoV-2 IgG. Overall seroprevalence among our study cohort for anti–SARS-CoV-2 IgG was 3.2% (Table 2). One of the patients who had a positive test result for SARS-CoV-2 RNA had a negative test result for anti–SARS-CoV-2 IgG (Table 3; patient 2). All patients with a positive test result (IgG or RNA) were contacted on August 10, 2020, and retrospectively surveyed for clinical symptoms of COVID-19 in the prior 6 months. Overall, only 3 patients (3 of 8 [37.5%], including the 2 patients we had identified by nasopharyngeal swabbing) recalled symptoms of flulike upper respiratory tract infection possibly related to COVID-19, whereas 5 did not. For 2 patients, we had temporally distinct serum samples available. Both showed a steep decline in antibody titers over time (Fig 2 ). One patient with a strongly positive test result for anti–SARS-CoV-2 IgG upon diagnosis had a formally seronegative test result by the end of the study (Fig 2 and Table 3; patient 1). All 3 clinically apparent COVID-19 cases were mild and self-limited. Patient 1, however, showed severe blood test abnormalities with severe thrombocytosis, coagulopathy, and elevated heart muscle enzymes.
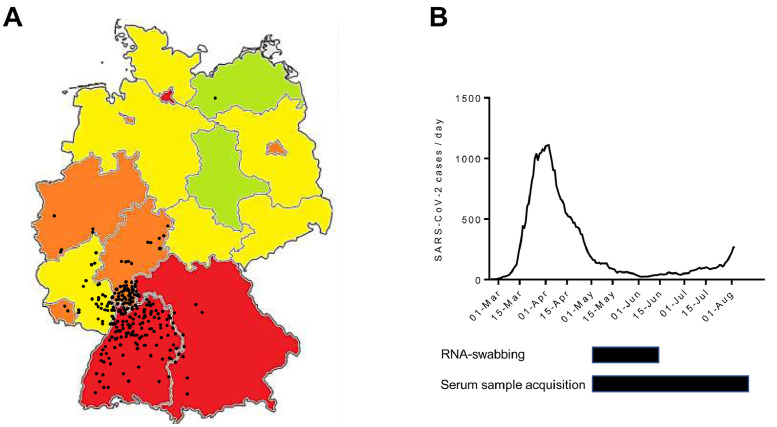
Fig 1.
The course of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in Germany. (A) Map of the Federal Republic of Germany indicating RNA-proven SARS-CoV-2 cases per capita in different federal states. Data as of August 15, 2020: red, >300 cases per 100,000; orange, 200-300 cases/100,000; yellow, 100-200 cases/100,000; green, <100 cases/100,000. Black dots indicate residence of enrolled LT recipients. (B) Number (7-day gliding average) of RNA-proven SARS-CoV-2 cases per day in the German state of Baden-Württemberg from February 25 until August 8, 2020. Below: Sample accrual time frame for nasopharyngeal RNA swab (upper black bar) and blood serum sample (lower black bar).
Fig 2.
Anti–severe acute respiratory syndrome coronavirus 2 (anti–SARS-CoV-2) serum titers in patients 1 and 7 over time.
Assessing the potential source of infection, of the seroconverted patients, 2 of 8 had potential contact with infected health care staff during a hospital stay. For the others, the source of infection was unknown. Interestingly, 2 of 8 recently underwent transplant, 2 of 8 were on dialysis for chronic renal failure, and 1 of 8 lived in an assisted living community.
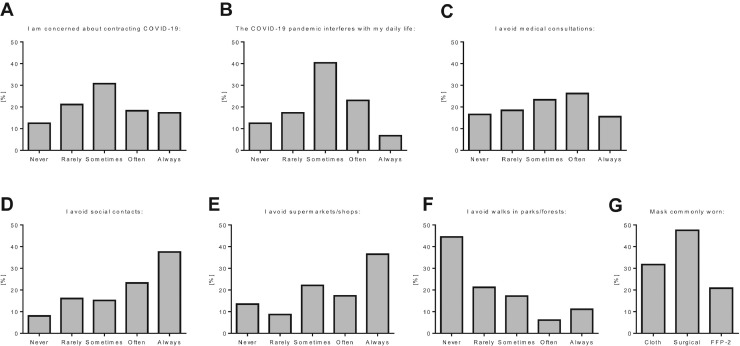
A total of 104 patients were surveyed at the time of sample collection for the use of protective equipment and personal behavior adjustments in the COVID-19 pandemic. Thirty-seven of 104 (35.6%) were highly or very highly concerned about contracting SARS-CoV-2, whereas 31 of 104 (29.8%) felt the pandemic highly or very highly interfered with their daily lives. Sixty-eight of 104 (65.4%) often or always avoided outside family social contacts, and 66 of 104 (63.5%) often or always avoided going to supermarkets or shops, but only 43 of 104 (41.3%) often or always avoided medical visits (Fig 3A-F). Most commonly worn face masks were surgical masks (47.5%), cloth masks (31.7%), and FFP2 filtering facepiece masks (20.8%) (Fig 3G). All patients always wore masks in supermarkets and in hospitals, and 16.3% always wore masks outside.
Fig 3.
(A-G) Survey of self-protection measurements and behavior adjustments among liver transplant recipients during the severe acute respiratory syndrome coronavirus 2 pandemic. COVID-19, coronavirus disease 2019.
Discussion
We performed a prospective screening trial for SARS-CoV-2 RNA and anti–SARS-CoV-2 IgG infection in LT recipients during the COVID-19 pandemic in southern Germany. We documented acute or past SARS-CoV-2 infection in 3.7% of our LT recipients during the study, which is in line with prior reports [15]. Although direct serosurveys in the general population are lacking for our study region, the SARS-CoV-2 infection percentage in the population can be roughly estimated from serosurvey studies in other regions with similar health care settings. Until study termination, about 37,000 PCR-proven SARS-CoV-2 infections had been documented in the study area (Baden-Württemberg). Recent German serosurveys provide a rough estimate of a factor of 4–10 of PCR-documented SARS-CoV-2 infections to overall serology-proven infections, proposing a seroprevalence of 1.3%-3.2% in southwestern Germany [16,17]. Taking into account this estimate, the prevalence of active or past SARS-CoV-2 infection in LT recipients is comparable to that in the general population. Our direct study was limited to 219 patients who presented for routine follow-up appointments after LT at our institution. However, the overall cohort comprises 1200 patients in the same region. These patients were encouraged to contact the transplant center in case of infection or hospital stay. Over the study period, we did not record a single case of hospitalization or death due to COVID-19 in this cohort. The presented data show an overall low but significant percentage of LT patients who had been infected with SARS-CoV-2. Five of 8 patients seroconverted without experiencing any clinical overt symptoms; in the other 3 of 8 patients, clinical symptoms were mild and self-limited. Interestingly, patient 2 (Table 3) had a positive screen for SARS-CoV-2 RNA but a negative result for anti–SARS-CoV-2 IgG on multiple occasions afterward. About 90% of non-immunocompromised patients develop anti–SARS-CoV-2 IgG after infection [18].
The detailed cases of patients 1 and 3 (Table 3) with repetitive serum samples highlight the potential rapid loss of anti–SARS-CoV-2 IgG within a few weeks. The extent to which immunosuppression adds to the phenomenon could not be assessed, owing to the overall low case number.
We further assessed how LT recipients changed their personal behavior using a standardized questionnaire. Most LT recipients were aware of the risk of SARS-CoV-2 infection and protected themselves with face masks and avoided public places such as supermarkets or social gatherings. Although a control group is certainly missing, it is surprising that, despite the self-containment efforts in this patient cohort, the SARS-CoV-2 IgG seroprevalence was comparable to that in the general population. Examining detailed patient information highlights the role that health care–acquired infection might play. Five of 8 patients were hospitalized during the pandemic or underwent hemodialysis for chronic renal failure. We cannot rule out that these patients contracted SARS-CoV-2 infection in the health care setting and that the health care system itself poses an important risk for this comorbid patient group. Concordantly, health care visits were less strictly avoided than supermarkets or social contact (Fig 3).
The present study has several important limitations. First, overall recorded numbers of seroconversion in the LT recipient cohort remained low, and although the specificity of the antibody test is given as 99.8% by the manufacturer, we cannot rule out false-positive test results. However, all positive test results were in the high titer range. Second, the study size was insufficient to assess individual risk factors such as immunosuppression, and it remains an important research question whether immunosuppression exerts protective effects against severe COVID-19 in LT patients. Compared with other studies that have highlighted increased morbidity and mortality in LT recipients with COVID-19, our study was conducted in a health care setting that was at no point overwhelmed during the first wave of the pandemic. Risk for severe COVID-19 in LT recipients seems comparable to that of the general population, and COVID-19 morbidity was low in a large LT recipient cohort. However, only 3.2% had antibodies against SARS-CoV-2, leaving potentially 96.8% of the cohort susceptible to SARS-CoV-2 infection. We documented a fast decline of anti–SARS-CoV-2 antibodies in two LT recipients, questioning the durability of anti–SARS-CoV-2 immunity and emphasizing the importance of continued containment efforts to protect at-risk patient groups.
Footnotes
The study was funded by the University Hospital Heidelberg.
References
- 1.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Ruiz M., Andrés A., Loinaz C., Delgado J.F., López-Medrano F., San Juan R., et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becchetti C., Zambelli M.F., Pasulo L., Donato M.F., Invernizzi F., Detry O., et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tschopp J., L’Huillier A., Mombelli M., Mueller N., Khanna N., Garzoni C., et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876–2882. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb G.J., Marjot T., Cook J.A., Aloman C., Armstrong M.J., Brenner E.J., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattar N., McInnes I.B., McMurray J.J.V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 13.Parente A, Manzia TM, Angelico R, Tirotta F, Muiesan P, Tisone G, et al. COVID-19, liver transplant, and immunosuppression: Allies or foes? Transpl Infect Dis [Internet]. 2020. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/tid.13417. Accessed January 8, 2021. [DOI] [PMC free article] [PubMed]
- 14.Ritschl P.V., Nevermann N., Wiering L., Wu H.H., Moroder P., Brandl A., et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A By-proxy Society Recommendation Consensus approach. Am J Transplant. 2020;20:1826–1836. doi: 10.1111/ajt.15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ossami Saidy RR, Globke B, Pratschke J, Schoening W, Eurich D. Successful implementation of preventive measures leads to low relevance of SARS-CoV-2 in liver transplant patients: Observations from a German outpatient department. Transpl Infect Dis [Internet]. 2020. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/tid.13363. Accessed November 9, 2020. [DOI] [PMC free article] [PubMed]
- 16.Santos-Hövener C, Neuhauser HK, Rosario AS, Busch M, Schlaud M, Hoffmann R, et al. Serology- and PCR-based cumulative incidence of SARS-CoV-2 infection in adults in a successfully contained early hotspot (CoMoLo study), Germany, May to June 2020. Eurosurveillance [Internet]. 2020;25(47). Available at: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.47.2001752. Accessed January 8, 2021. [DOI] [PMC free article] [PubMed]
- 17.Streeck H., Schulte B., Kümmerer B., Richter E., Höller T., Fuhrmann C., et al. Infection fatality rate of SARS-CoV2 in a super-spreading event in Germany. Nat Commun. 2020;11:5829. doi: 10.1038/s41467-020-19509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]