Abstract
COVID-19 virus is classified as a respiratory disease that can be mainly transmitted via respiratory droplets, however, there are recently published reports suggested its ability to transmit via sexual intercourse, assisted reproductive technology (ART) treatments, pregnancy, and nursing. Although SARS‐CoV‐2 mainly attacks respiratory systems, manifestations of multiple organs have been detected. A significant concern was raised about whether COVID‐19 may affect female and male reproductive functions. These findings imposed more restrictions on social relationships between individuals even if inside the family, adding more physiologic load. In this context, there is a crucial need to identify the biological and behavioral reproductive risk factors associated with COVID19 disease. Questions regarding the potential risks of sexual transmission during intercourse and/or application of ART, vertical transmission (throughout pregnancy, delivery, and breastfeeding), the health of pregnant and postpartum women, and fetal or postnatal health problems of neonates/children remain largely unanswered. The contribution of individuals to different social and economic activities depends on the maintenance of good quality life and health. The ongoing COVID-19 pandemic raised on the end of December 2019 has drastically affected different aspects of human wellbeing. The pandemic not only affected the health of individuals, but also negatively affected mental health and social interaction. This review illustrates: a) scientific findings related to the impact of the COVID-19 pandemic on the reproductive process, considering gender, hormonal balance, gonad functions, pregnancy, and ART, b) the sociosexual dimension of COVID-19 disease and precautions that should be taken to avoid infection via sexual transmission or vertical transmission, which may alleviate the fear associated with continuing normal social relationships and economic activities.
Keywords: COVID-19, Gender, Hormones, Sexual disease transmission
List of abbreviations
- ART
Assisted reproductive technology
- ASRM
American Society for Reproductive Medicine
- ESHRE
European Society for Human Reproduction and Embryology
- LH
Luteinizing hormone
- PCOS
Polycystic ovary syndrome
- RA
Reis AM
- StAR
Steroidogenic acute regulatory
- WHO
World Health Organization
1. Introduction
In December 2019, Chinese authorities announced the appearance of many cases of pneumonia, some resulting in mortality, in Wuhan Province, triggered by an unidentified viral disease (WHO). Many other similar cases have subsequently been observed worldwide, with a massive number of deaths. Against this background, virologists isolated and identified the new causative virus. This virus was shown to be a member of the beta-coronaviruses and was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The associated disease was named coronavirus disease (COVID-19) (Rodriguez-Morales et al., 2020). Within only 3 months of its appearance, on 11/03/2020, the rapid spread of the virus led the WHO to announce a public health emergency and classify the situation as a global pandemic. The sudden emergence of COVID-19 disease has drastically changed economic activities and social relations globally (Hashem et al., 2020). These changes in activities are due in part to the local and global restrictions on movement imposed by various authorities and the rising fear of contagion (Ibarra et al., 2020). The situation has been exacerbated by the fact that the behavior of this new virus has remained largely unclear. Only a few scientific findings have revealed its consequences for human health and quality of life, including sexual and reproductive health and wellbeing. In this context, it should be borne in mind that reproduction is not only an innate biological function for producing new individuals, but also has many social and physical dimensions. A good quality sex life with frequent safe sex is necessary for achieving adequate physical, mental, and social health (Yuksel, and Ozgor, 2020). Thus, there is a crucial need to identify the biological and behavioral reproductive risk factors associated with COVID-19 disease. Specifically, previous experiences with viral diseases such as zika, ebola, hepatitis viruses B and C, and human immunodeficiency virus have shown the possibility of sexual disease transmission via the semen of infected individuals during intercourse. Furthermore, many viruses such as influenza, mumps, HIV, and zika can induce testicular orchitis, causing male reproductive dysfunction and infertility (Liu et al., 2018). Another concern associated with COVID-19 disease and reproductive functions is the risk of vertical transmission (from the pregnant mother to the fetus) (Tang et al., 2020, Chen et al., 2020). Questions regarding the potential risks of vertical transmissions, such as the mode/timing of transmission (throughout pregnancy, delivery, and breastfeeding), the health of pregnant and postpartum women, and fetal or postnatal health problems of neonates/children remain largely unanswered (Siston et al., 2010, Wong et al., 2004, Tang et al., 2020). The same fear extends to couples who intend to use assisted reproductive technology (ART). These couples are in a particularly difficult situation as they already suffer from infertility and would thus generally be anxious about their fate (de Souza et al., 2020). At many ART centers, fertility treatments have been postponed in association with the COVID-19 pandemic, placing great pressure on couples for whom age is a critical factor (Vaiarelli et al., 2020).
From the social perspective, it is expected that COVID-19 will have various negative sociosexual impacts compared with previously emerging humanitarian crises, given the findings of a few recent reports (Arafat et al., 2020, de Souza et al., 2020, Fan et al., 2020). Restrictions on human movement and activity generally reduce access to clinical reproductive healthcare support and reproductive healthcare products, which has many negative social and reproductive/mental health-related repercussions. For example, restricted access to contraception can increase the rate of unplanned pregnancy, which can be related to pregnancy complications, unsafe abortions, postpartum depression, and even suicide. In addition, a lack of reproductive hygiene products can facilitate sexually transmitted infections (Rasmussen et al., 2020).
This review illustrates scientific findings related to the impact of the COVID-19 pandemic on the reproductive process, considering gender, hormonal balance, gonad functions, pregnancy, and ART. In addition, the sociosexual dimension is discussed, in terms of precautions that should be taken to avoid infection via sexual transmission or vertical transmission, which heightens the fear associated with conducting normal social relationships.
2. Viral receptor expression through the reproductive organs
The initial step of COVID-19 infection relies on attachment between viral spike glycoprotein receptors and the angiotensin converting enzyme 2 receptor (ACE2, a zinc metalloprotease) on the surface of the host cells. The completion of viral entry into host cells requires further conformational changes of the viral spike protein, which is mediated by host cell proteolytic enzymes, mainly transmembrane serine protease 2 (TMPRSS2) (Jing et al., 2020, Stanley et al., 2020).
Along the female genital tract, the expression of both ACE2 and TMPRSS2 has been confirmed in different reproductive organs, including the ovary (stroma and different germ cells), uterus, vagina, and placenta, with varying expression levels and specific functions (Jing et al., 2020, Stanley et al., 2020). In the ovary, ACE2 is expressed in different ovarian cells of mammals such as rats (stroma, granulosa cells, and oocytes; Pereira et al., 2009), bovines (theca cells and granulosa cells), and women of reproductive age as well as postmenopausal women (Tonellotto dos Santos et al., 2012, Barreta et al., 2015). In humans, ACE2 is expressed in the ovaries, while angiotensin-1–7 are found at detectable levels in ovarian follicular fluid (Reis et al., 2011). In addition, ACE2 is highly expressed in human germ cells and early-stage embryos (Yan et al., 2013). In ovarian cells of humans and non-human primates, ACE2 is widely expressed, while TMPRSS2 expression is very limited. Notably, the coexpression of ACE2 and TMPRSS2 tends to increase in ova with the progress of folliculoogenesis, as ova in primordial follicles were reported to possess minimal coexpression, while 62% of those in atretic follicles possess detectable expression of both ACE2 and TMPRSS2 (Stanley et al., 2020). Biologically, ACE2 controls many pivotal physiological functions in the ovary, such as folliculogenesis, follicular atresia, and ovulation (Obermüller et al., 2004, Guo et al., 2018), luteal angiogenesis and luteolysis (Sugino et al., 2005), and steroid secretion (Hayashi et al., 2003, Costa et al., 2003). In terms of the uterus, the expression of ACE2 has also been confirmed in the human uterus (Vaz-Silva et al., 2009) and rat uterus (Brosnihan et al., 2012), being more abundant in uterine epithelial cells than in stromal cells, and exhibiting a higher level in the secretory phase than in the proliferative phase (Vaz-Silva et al., 2009). Ang II plays several roles related to uterine structure and function, including regeneration and remodeling of the endometrium, regulation of myometrium contractile activity (Vaz-Silva et al., 2012), and maintenance of regular menstrual cycles (Li and Ahmed, 1996). In the placenta, ACE2 has been identified in placental microvilli, cytotrophoblasts, syncytiotrophoblasts, and endothelium, as well as vascular layers of smooth muscle. It is also expressed in the maternal stroma (trophoblast and decidual cells) as well as the umbilical cord (venous endothelium and smooth muscle) (Valdés et al., 2006, Valdés et al., 2013). Interestingly, Jing et al. (2020) reported that the expression of ACE2 in the placenta is greater than that in the lung, suggesting that the placenta could be an organ through which COVID-19 viral infection occurs. The same could be expected for all reproductive organs expressing ACE2, leading to many reproductive dysfunctions and reproductive infertility.
In terms of the male reproductive tract, it has been documented that ACE2 expression is much higher in the genital tissues of the male reproductive tract than in the female reproductive tract (Pan et al., 2013). Various studies have confirmed the abundant expression of ACE2 receptors in different somatic and germ testicular cells, principally in Sertoli cells, Leydig cells, and seminiferous duct cells, as well as spermatogonia (Fan et al., 2020, Shen et al., 2020, Wang and Xu, 2020). Consequently, it is strongly suspected that testicular injury and infertility could occur due to COVID-19 infection (Abobaker and Raba, 2020, Verma et al., 2020).
3. Distribution of covid-19 between the sexes
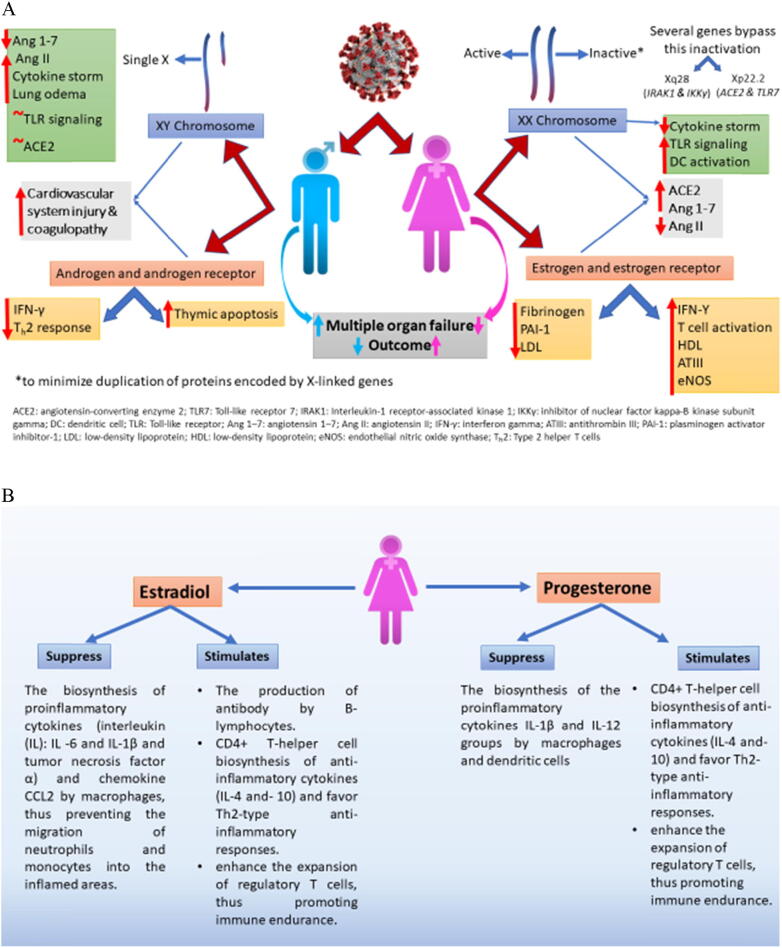
In the epidemiology of COVID-19 infection, the frequency of infected males and their fatality rates are surprisingly considerably higher than those recorded for females. This has been recorded in many countries (China, South Korea, and Italy) worldwide and is mainly ascribed to sexspecific biological susceptibility (Global Health 5050). For instance, in China, about 51%–66.7% of affected patients were reported to be men (Mo et al., 2020; Chen et al., 2020). Similarly, in Italy, about 58% of cases and 70% of COVID-related deaths were of men (Remuzzi and Remuzzi 2020). Other proposed reasons for this is a discrepancy between the sexes include variation in preexisting comorbidities, behavioral risk factors, and the overlap between these key factors (Chen et al., 2020, Remuzzi and Remuzzi, 2020). From a biological perspective, there are several biological and evolutionary factors enabling females in particular to manifest robust immune responses against different pathogens, including viruses. These might explain their lower infection rate and fewer fatal outcomes from different infectious diseases including COVID-19. In an early study, it was confirmed that women can produce higher levels of circulating antibodies, specifically immunoglobulins IgG and IgM, than men (Butterworth et al., 1967). Furthermore, compared with males, females can also develop high levels of immune cells such as CD4+ T helper cells (Amadori et al., 1995). Genetically, females have more than 60 immune-response genes due to their possession of two X chromosomes, versus only one in males (Klein; Kloc et al., 2020). In this context, ACE2 receptors, the main host receptors for COVID-19 virus, are encoded by the ACE2 gene sited on the X chromosome. It was supposed that some of the alleles of this gene can encode receptors with different viral recognition and viral binding efficiency. However, all male cells constantly express a single ACE2 allele because all cells have and express the same X chromosome. This is not the case in females because of the mosaicism of their X chromosomes, the expression of which is randomly allocated among the cells (Kloc et al., 2020). Accordingly, in females, a more efficient form of ACE2 receptor could potentially appear in half of the cells. This could explain the limited infectivity of coronaviruses such as SARS-CoV-1 and SARS-CoV-2 in females and the resistance of females to infection.
Sex steroid hormones, P4 and E2, which play pivotal immunomodulatory roles, can also aid the resistance of females. For example, variations in the levels of E2, P4, and androgens between females and males have been found to influence both the COVID-19 infection rate and the intensity of COVID-19-associated symptoms. In this regard, Mauvais-Jarvis et al. (2020) postulated that the anti-inflammatory and immunomodulatory mechanisms of E2 and P4 as well as their effects on renin-angiotensin system (RAS) could be related to the rate of COVID-19 infection. Estrogens have a significant role in regulating RAS expression and activity in different organs including reproductive organs, however its effects depend on many factors including type of estrogen receptors and its distribution, sex and physiological status, and pathological status. A growing body of evidence showed the sexual dimorphic pattern of estrogens on cardiovascular disease via regulating RAS, however the relation between modulatory effect of estrogens on RAS and the rate of COVID-19 infection in both sexes remains unclear (Groban et al., 2020). High concentrations of E2, parallel to those during ovulation and pregnancy, inhibit the biosynthesis of several proinflammatory cytokines [interleukin (IL)-6, IL-1β, and tumor necrosis factor α] and the chemokine CCL2 by macrophages, thereby preventing the migration of phagocytes, neutrophils, and monocytes into inflamed areas. Furthermore, E2 stimulates the biosynthesis of antibodies by B-lymphocytes. In addition, P4 inhibits the biosynthesis of other groups of proinflammatory cytokines such as IL-1β and IL-12 by macrophages and dendritic cells. A high concentration of either E2 or P4 stimulates the biosynthesis of anti-inflammatory cytokines (IL-4 and IL-10) by CD4+ T helper cells, evoking Th2-type-mediated anti-inflammatory responses. Additionally, the expansion of regulatory T cells can be enhanced by the action of both steroids, promoting immune tolerance. In contrast, many findings have suggested the ability of androgens (testosterone) to amplify the intensity of COVID-19 symptoms. Indeed, androgens have been suggested to activate the ACE2 pathway, facilitating the entry of COVID-19 into host cells (La Vignera et al., 2020). These findings highlight the possibility of using P4 and E2 agonists (Mauvais-Jarvis et al., 2020, Klein and Flanagan, 2016) and androgen antagonists (Goren et al., 2020) as potential therapies against severe COVID-19 symptoms, specifically those related to cytokine storm, which is the primary cause of mortality in severe cases in other respiratort diseases such as Severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) (Mauvais-Jarvis et al., 2020, Klein and Flanagan, 2016). However, further robustly designed studies are needed to confirm or reject the outcomes of sex steroid-based therapy for tackling COVID-19 symptoms. The role of sex chromosomes (A) and sex steroid hormones (B) in distribution of covid-19 between the sexes are summarized in Fig. 1.
Fig. 1.
Role of sex chromosomes (A) and sex steroid hormones (B) in distribution of covid-19 between the sexes.
4. Potential of sexual transmission and impacts on sexual behavior
It has been confirmed that COVID-19 can be transmitted by human-to-human contact via respiratory droplets when individuals are up to two meters apart. Infection may also take place after touching an infected surface followed by touching the mouth, nose, or eyes (Stadnytskyi et al., 2020). Moreover, clinical research has reported that other routes of contagion may occur, such as via contact with the blood (Yu et al., 2020), feces (Cheung et al., 2020), or semen (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c) of an infected person.
Sexual transmission is a major concern for couples wishing to conceive and pregnant women. Notably, several reports have confirmed a high incidence of infection among sexual partners of COVID-19-positive females (Cui et al.,2020), suggesting the possibility of sexual transmission. To investigate this issue, COVID-19 virus was isolated from saliva, feces, and semen samples of infected individuals (Zhu et al., 2020, Zhou et al., 2020). The largest amount of virus was present in saliva, and thus could be transmitted via the saliva during the physical contact between couples. However, semen samples of COVID-19-infected men showed positive results in a COVID-19 test, but there is as yet no evidence that COVID-19 can be transmitted via semen during vaginal or anal intercourse (Ibarra et al., 2020). In another study, Wu et al. (2020) claimed that one of three samples of breast milk obtained from COVID-19 infected pregnant women in the third semester of pregnancy was positive for COVID-19, indicating the possibility of transmission through breastfeeding. Even if there is no virus in milk, transmission via close contact between the mother and neonate during breastfeeding through arousal transfer should be taken into account. The transplacental transmission of SARS-CoV-2 infection is possible during the last weeks of pregnancy which may cause placental inflammation, neonatal viremia and neurological symptoms due to cerebral vasculitis (Vivanti et al., 2020).
As a tactic to limit the spread of COVID-19, the WHO (2020) suggested personal preventive actions (cleaning/disinfection of the environment, hand/respiratory hygiene, facial coverings, and cough etiquette) and social preventive measures, such as physical/social isolation and staying at home. Although these safety measures lead to social isolation, they could impede the COVID-19 pandemic. In this context, sexual contact principally between those who do not live together has been discouraged because it might aid the spread of COVID-19 (Arafat et al., 2020). Sexual behavior is a term that includes a wide spectrum of biological functions and psychological actions. It is expected that the emergence of a major health crisis such as the COVID-19 pandemic would greatly affect sexual behavior at both biological and psychological levels. This is particularly true because stress is known to impact female sexual desire and frequency of sexual intercourse. In this term, a survey study carried out by Yuksel and Ozgor (2020) revealed that, during the COVID-19 pandemic, there were significant decreases in quality of sex life, intention to bear a child, and female access to contraception, as well as an increase in menstrual disorders.
5. Impacts of covid-19 on gonad functions
The fact that ACE2 receptors are expressed by different endocrine glands, including those directly (testis, ovary, and pituitary) or indirectly (pancreas, thyroid, and adrenal glands) controlling reproductive functions and higher reproductive centers in the brain, highlights the potential impacts of COVID-19 on different reproductive events (Pal and Banerjee, 2020).
5.1. Ovary functions
There is currently inadequate information on ovarian function in women infected with COVID-19 virus (Pal and Banerjee, 2020). One of the few reports published on this issue discussed the association between a common ovarian dysfunction in women, namely, polycystic ovary syndrome (PCOS), and the incidence of severe COVID-19 infection and/or symptoms (Kyrou et al., 2020). The value of this study is ascribed to the fact that PCOS may account for about 10%–15% of endocrine disorders in women (Teede et al., 2018), and that about 75% of women suffering PCOS also have obesity and other pre-existing health problems such as type 2 diabetes and hypertension. Given the ability of the sex steroid hormones to modulate the expression and activity of ACE-2 in many tissues such as adipose tissue, myocardium, and kidney, women with POCS are highly likely to suffer severe adverse symptoms of COVID-19. Specifically, women with POCS usually have high concentrations of circulating androgens, which are strongly suggested to facilitate COVID-19 entry (La Vignera et al., 2020, Kyrou et al., 2020). Women suffering any comorbidities accompanying ovarian dysfunction should be made aware of the hazards of COVID-19 infection. They should also receive sufficient healthcare monitoring by professionals to avoid any possible pathological complications. In this term, therapies including vitamin D as one of the therapeutic protocol may present an effective measure to decrease the severity of COVID-19 symptoms and the infection rate of COVID-19 in POCS women. It has been confirmed that women with PCOS have a high level of vitamin D deficiency. Hence, a correlation has been established between the serum vitamin D level and several metabolic symptoms in PCOS patients. Vitamin D deficiency may be responsible for the pathogenesis of PCOS (Fang et al., 2017). Vitamin D supplementation may be beneficial for follicular development and menstrual cycle regulation in patients with PCOS. Many studies have confirmed that women with PCOS may be at potentially higher risk for severe COVID-19 due to low vitamin D levels as one of the associated POCS symptoms. Furthermore, there is an association between vitamin D deficiency and the severity of PCOS symptoms, including infertility, hyperandrogenism, insulin resistance, and cardio-metabolic disease (Reis et al., 2017, Azadi-Yazdi et al., 2017).
5.2. Testis functions
In males, COVID-19 virus can affect male fertility through increasing the body temperature and subsequently the incidence of fever (Fan et al., 2020). Around 80% of COVID-19 patients were reported to exhibit sustained increases in body temperature (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c). It has also been suggested that fever and the increase of patients’ testicular temperature can damage spermatogenesis and thus male fertility (Younis et al., 2020).
Intriguingly, the testicular expression of ACE2 is associated with age (Shen et al., 2020), as the highest level of ACE2 receptors was documented in patients in their 30 s, while 60-year-old patients exhibit the lowest level (Shen et al., 2020). A higher expression of ACE2 was observed in patients aged 20–30, whereas as 60+ -year-old patients showed a reduced expression of ACE2. This might suggest that youthful male patients are at higher risk of testicular impairment by COVID-19 than older patients (Navarra et al., 2020).
In previous work, an assessment of autopsy specimens of the testis of six patients who died due to SARS-Cov infection in 2002 demonstrated an indication of orchitis (Xu et al, 2006). It was also reported from a histopathological analysis that SARS-Cov infection showed inflammatory infiltrates, principally in seminiferous ductal cells (Xu et al., 2006). This might support the hypothesis that inflammatory responses causing testicular injury are due to immunological and inflammatory responses rather than direct destruction by the virus infection. Here, it can be speculated that COVID-19 infection has the potential to cause testicular destruction and subsequent infertility. In other words, it could be suggested that testicular deterioration could potentially be triggered by either direct viral infection via the attachment of COVID-19 virus to ACE2 receptors or indirectly by secondary inflammatory and immunological reactions. However, that COVID-19 could have pathophysiological influence on testicular function was proposed based on further documents demonstrating that active COVID-19 infection dramatically decreased the testosterone/luteinizing hormone (LH) ratio, demonstrating a notable influence on the responsiveness of Leydig cells to LH stimulation (Wang et al., 2020). Moreover, COVID-19 generated alterations in the cytokine profile that may have further consequences for male fertility (Loveland et al., 2017). Follow up research on reproductive regulation in male patients who have recovered from COVID-19 infection is needed to explore this possibility.
It was reported that ACE2 exerts significant function during spermatogenesis (Fan et al., 2020). Several reports have been published on male reproductive damage after COVID-19 infection, with associated effects on male reproductive health. It has been hypothesized that such damage is associated with an immune-mediated response to infection, although direct inoculation of the COVID-19 virus within testicular cells has not been described. Previously, based on data collected from postmortems in a 2005 study of eight patients affected by SARS-CoV, testicular cells were shown to suppress focal atrophy despite a deficiency in detectable SARS viral RNA (Gu et al., 2005). Consequently, it has been described that SARS-CoV virus could trigger acute orchitis, as supported by the massive precipitation of immunoglobulin (Ig)G in testicular tissue, leading to prevalent testicular leukocyte infiltration and germ cell damage (Xu et al., 2006).
Like ACE2, Mas mRNA is highly expressed in the testis, especially in Leydig and Sertoli cells (Shen et al., 2020). Similarly, recent work has shown that human spermatozoa also express the angiotensin-1–7 Mas receptor (Leal et al., 2009). For instance, the receptor Mas has been discovered in rat and mouse testis; its level begins to rise during puberty and its expression peaks during the reproductive period. Knockout in mammals, particularly that of several elements of RAAS such as Mas knockout mice, demonstrated abnormal expression of genes participating in testicular steroidogenesis and mitochondrial function (Leal et al., 2009, Shen et al., 2020).
Nevertheless, unlike the status for alveolar cells, it has not yet been established whether cells participating in spermatogenesis are dependent on intact ANG1–7 for functional integrity, which can be explored using appropriate techniques.
Recently, the transcript level of ACE2 in the testis of adult humans in a number of single-cell transcriptomes was demonstrated to be mainly increased in Sertoli and Leydig cells, as well as in spermatogonia (Shen et al., 2020, Verma et al., 2020). Also, Mas and Ang-1–7 were discovered in the interstitial compartment and the seminiferous tubules mainly in Leydig cells, in males with regular spermatogenesis development (Valdivia et al., 2020, Leal et al., 2009).
However, neither element of the renin-angiotensin-aldosterone system (RAAS) was observed in the seminiferous ducts of sterile males with non-obstructive azoospermia (Reis et al., 2010). Taking these findings together, RAAS, and precisely ACE2, appears to perform an essential function in male reproductive regulation. The collected data imply that the RAAS elements participate in human male regulation of testosterone synthesis, steroidogenesis, and spermatogenesis in the testicular tissues (Aitken, 2020). However, it is also likely that the virus could gain entry to male germ cells once they leave the testes, either in the epididymis or following ejaculation. As such, it is thought that the mature spermatozoa have all of the machinery required to attach to this virus (COVID-19), combine with it, and even achieve reverse transcription of the viral RNA into pro-viral DNA. These issues increase the probability that spermatozoa could be vectors of this highly contagious disorder (Aitken, 2020). This happens in insects (Mao et al., 2019), so it could also happen in humans.
For several years, it has been accepted that ACE is highly expressed on the surface of human sperm. Investigations of proteomic databases (Castillo et al., 2018, Wang et al., 2016) as well as studies of the sperm surface with monoclonal antibodies (Valdivia et al., 2020) showed that these cells generally possess all of the ACEs.
Endothelial dysfunction, subclinical hypogonadism, psychological distress and impaired pulmonary hemodynamics contribute to the potential onset of erectile dysfunction. Additionally, COVID-19 might exacerbate cardiovascular conditions; therefore, further increasing the risk of erectile dysfunction. Testicular function in COVID-19 patients requires careful investigation for the unclear association with testosterone deficiency and the possible consequences for reproductive health. Treatment with phosphodiesterase-5 inhibitors might be beneficial for both COVID-19 and erectile dysfunction.
Actual fusion between human spermatozoa and virus requires the presence of the above-mentioned protease, TMPRSS2, to cleave the viral spike proteins (S) at the S1/S2 boundary or within the S2 subunit, thus eliminating the structural restraint of S1 on S2 and releasing the internal membrane fusion peptide (Aitken, 2020). Chen et al. (2020) suggested that this protein acts in prostasomes that are synthesized in the prostate gland and released into seminal fluid upon ejaculation (Chen et al., 2020).
As one of the main functions of these exosome-like structures is to relocate their contents, proteins, to the spermatozoa following ejaculation, the incorporation of TMPRSS2 from this source seems probable (Aitken, 2020, Chen et al., 2020). Moreover, a close examination of human sperm proteomic data showed the existence of associated proteases TMPRSS127 and TMPRSS11B as well, which are supposed to act as proteases stimulating viral infection including COVID-19 (Ji et al., 2020, Singh et al., 2020). The existence of these stimulating proteases as well as ACE2 in the plasma membrane of spermatozoa would be anticipated to enable the COVID-19 virus to attach to the cell surface and eventually fuse to it, either in the testes or during the prolonged localization of these cells in the epididymal canal.
In conflict with this, oocytes appear to be entirely lacking TMPRSS2 protein (Singh et al., 2020), making infection of the female germ cells highly unlikely, unless, of course, they are fertilized by a COVID-19-carrying spermatozoon. In this situation, it should be highlighted that spermatozoa have a notable capacity to transmit viral infections from the male genitals to the female reproductive tract, as happens during the sexual transmission of the zika virus (Joguet et al., 2017). Studies have also demonstrated that the abilities to combine with viruses in the surroundings (Nussbaum et al., 1993, Aitken, 2020) and acquire reverse-transcriptase activity are accomplished by the production of pro-viral DNA (Aitken, 2020), as is evidently the case for human immunodeficiency virus 1. Regarding the steroidogenic hormones in COVID‐19 patients, it was reported that COVID‐19 significantly increased the serum LH compared with that in healthy people (Ma et al., 2020). Even though there were no significant differences in serum testosterone and FSH levels between the two groups, the ratios of testosterone: LH and FSH: LH were significantly decreased in the COVID‐19 group (Ma et al., 2020). The observation of hormonal disturbances of COVID‐19 patients might be associated with virus‐induced inflammation causing the local or systematic release of cytokines. The elevated secretion of cytokines may damage testicular cells and thus impede testosterone secretion by repressing the transcription of the rate‐limiting enzyme steroidogenic acute regulatory protein (StAR) (Lin et al., 1998). Furthermore, the reduction in testosterone secretion stimulates LH release, which can sustain testosterone level in the short term (Ma et al., 2020). Testosterone replacement therapy (TRT) is largely beneficial in the treatment of hypogonadal men. TRT has known harmful effects if inappropriately prescribed, and a meta-analysis study did not find any conclusive evidence of a potentially therapeutic effect of testosterone administration, neither acute nor chronic, on endothelial function (Sansone et al., 2019). It would be reasonable to perform further experiments on the reproductive features associated with COVID-19 infection, especially in recovered patients.
Available data on male sexual endocrine function revealed that men infected with COVID-19 had lower serum testosterone (T) concentrations, higher LH concentrations, and lower T:LH ratios. These hormonal imbalances can negate the possibility of suppression of the hypothalamic–pituitary–testicular axis, driving primary Leydig cell damage (Ma et al., 2020). Impairment of spermatogenesis was observed in COVID-19 patients, which could be partially explained as a result of an elevated immune response in testis. Additionally, autoimmune orchitis occurred in some COVID-19 patients. A series of studies have provided the negative influence of COVID-19 infection in spermatogenesis through impairment the immune responses (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c), abnormal sex hormone secretions (Ma et al., 2020). Additionally, autoimmune orchitis occurred in some COVID-19 patients (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c). From the pathological screening, it was observed that testes from COVID-19 patients displayed significant seminiferous tubular injury, mild lymphocytic inflammation and reduced Leydig cells (Yang et al., 2020). Further research on the reversibility of impairment and developing treatment are warranted.
6. Semen quality
Although COVID‐19 primarily assaults the respiratory tissues, indications of the involvement of various tissues have been detected. Concerns were thus raised about whether COVID‐19 can affect male reproductive health. In a cohort study by Li et al., 2020a, Li et al., 2020b, Li et al., 2020c, it was observed that COVID-19 can appear in the semen of infected individuals and may even be present in the semen of recovering patients. Because of the deficient epididymis barrier/deferens/blood-testes barrier, COVID-19 might be scattered throughout the male reproductive system, mainly in the form of systemic local infection. Surveys on viral recognition and semen perseverance would be valuable for clinical practice and public health, especially regarding viruses that could trigger high morbidity or mortality, such as COVID-19. It has been reported that 24% of 38 patients infected with COVID-19 were incapable of providing a semen specimen because of erectile dysfunction, dying prior to recruitment, or being in a comatose state (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c).
Several research groups reported that no COVID-19 was detected in semen samples from patients (Ma et al., 2020; Pan et al., 2020; Guo et al., 2018, Paoli et al., 2020, Song et al., 2020), suggesting the protecting role of the blood‐testes barrier against COVID‐19 (Wang et al., 2020). Anyway, since most of the semen samples in the survey of Ma et al. (2020) were from patients in the recovery phase, the virus (if it ever existed in semen) may have been cleared by the time of analysis. Ma et al. (2020) reported that 66% (8/12) of patients had normal semen attributes. However, 33.3% of patients had low sperm motility with a high sperm DNA fragmentation index (20.05 ± 3.80 vs. 7.6 ± 2.2 in the other eight patients), among whom two patients also had inferior sperm morphology and three described a deficiency of libido after the COVID‐19 outbreak. One case also reported the failure of morning erection (Ma et al., 2020). However, many reports have mentioned that COVID-19 patients had a regular range of semen attributes after the first discovery of infection (Guo et al., 2018, Paoli et al., 2020). Specifically, semen attributes were categorized in 12 patients, and eight contributors had regular semen traits after COVID‐19 infection (Ma et al., 2020). An increased sperm DNA fraction ratio was only observed in four patients infected by COVID-19 with inferior semen traits. Among three men who underwent semen analysis before COVID‐19 infection, the total sperm motility in two cases showed a minor decline in comparison to their prior recorded values (Ma et al., 2020). However, owing to the inadequate sample scope and the huge biological alteration in semen quality variables, a further well‐designed cohort study is required to clarify the potential effects of COVID‐19 on spermatogenesis. Regarding the age effect, young men are at higher risk of testicular damage by the COVID-19 than old men. Because the testicular expression of ACE2 is higher in men aged 20–30 than older men. Additionally, men aged 60+ -year-old showed a reduced expression of ACE2. Therefore, it can be concluded that expression of ACE2 is age-related.
7. Impacts on pregnancy
Many reports including those on clinical studies showing the potential health risks of COVID-19 during pregnancy have been published, with conflicting results (de Souza et al., 2020, Fan et al., 2020, Chen et al., 2020, Dong et al., 2020, Zeng et al., 2020). For example, among most COVID-19 pregnancies (greater than20 weeks) followed in China, so far, there has been a lack of vertical transmission (maternal–fetal transmission during pregnancy) of COVID-19 virus (Souza et al., 2020). Similarly, Fan et al. (2020) ruled out the possibility of vertical transmission of COVID-19 virus. However, cases of COVID-19 vertical transmission, particularly in late pregnancy, have been reported elsewhere (Chen et al., 2020, Liu et al., 2020, Zhu et al., 2020), and subsequent infections 2 or 4 days postpartum were identified in newborn infants who were subjected to nasopharyngeal and anal swab tests (Zeng et al., 2020). In addition, in another report, newborn infants born to COVID-19-infected mothers had increased levels of IgM antibodies, only at 2 h after delivery (Dong et al., 2020). In this context, Zeng et al. (2020) reported that about 9% (3/33) of newborn infants born to mothers bearing COVID-19 were infected via intrauterine vertical transmission. These findings support the potential for the vertical transmission of COVID-19 virus during pregnancy. Thus, caution should be taken among couples who plan to have a child or women who are already pregnant to avoid potential health risks to both mother and child.
During pregnancy, the balance among Ang II, ACE2, and Ang-1–7 controls the maintenance of pregnancy by regulating maternal hypertension and fetal development. These molecules can also control embryo implantation (Ghadhanfar et al., 2017), placental blood, nutrient supply to fetuses (Shibata et al., 2006, Anton et al., 2008, Anton et al., 2009), and intrauterine growth restriction (Ghadhanfar et al., 2017). Studies using either single-nucleotide RNAseq or single-cell RNAseq have confirmed the placental expression of ACE2, but with either no or very low expression of TMPRSS2 (Pique-Regi et al., 2020, Sungnak et al., 2020). Immunohistochemical analysis of placental tissue confirmed the existence of ACE2 in many types of placental cells, such as syncytiotrophoblasts, cytotrophoblasts, endothelium, and vascular smooth muscle (Valdés et al., 2006, Valdés et al., 2013). Interestingly, ACE2-mediated pathways can control trophoblast migration, vascular remodeling, and maternal vasodilation (Pringle et al., 2011, Valdés et al., 2006, Valdés et al., 2013). The disturbance in ACE2-mediated pathways might be the cause of pregnancy failure by inducing miscarriage, ectopic pregnancy, and preeclampsia (Valdés et al., 2006, Valdés et al., 2013). Therefore, if COVID-19 virus changes the expression of ACE2 in the placenta, there is a potential risk for the occurrence of placental malformation and many other pregnancy-related complications. These assumptions could be confirmed by the results of published studies in this field. From the available literature, women infected with COVID-19 have a risk of premature birth, fetal distress, preterm premature rupture of amniotic sac, and cesarean delivery (C-section) (Chen et al., 2020, Ferrazzi et al., 2020, Li et al., 2020a, Liu et al., 2020, Zeng et al., 2020, Zhu et al., 2020). Supporting these findings, Shanes et al. (2020), who examined the morphology of the placentas of 16 COVID-19-infected patients, revealed the occurrence of placental morphological abnormalities, mainly maternal vascular malperfusion (decidual arteriopathy, fibrinoid necrosis, and fetal vascular malperfusion). Surprisingly, the maternal vascular malperfusion features observed in the placentas of those patients were not associated with hypertensive, acute inflammatory, or chronic inflammatory pathology. This suggests that the placenta could be a organ that is sensitive to the effects of COVID-19 and highlights the role of other infection mechanisms, rather than an inflammatory storm. Thus, such infection is expected to have adverse outcomes on the functions of placenta and fetal intrauterine growth (Shanes et al., 2020, Golden and Simmons, 2020).
Regarding the risk to maternal health at pregnancy, so far, the evidence of two cross-sectional studies have shown that the symptoms accompanying COVID-19 infection during early pregnancy are not more severe than those among non-pregnant women (Li et al., 2020a, Li et al., 2020b, Li et al., 2020c). Nonetheless, some infected pregnant patients have suffered very serious respiratory symptoms, most of who gave birth immediately after the expression of these severe symptoms, whether premature or at full term. In addition, adverse outcomes regarding the health of newborn infants have been reported; however, enough is not yet known about whether these outcomes were related directly to COVID-19 infection (Segars et al., 2020).
8. Impacts on assisted reproductive technology
Globally, different fertility societies, such as the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM), have called to postpone most ART applications, including ovarian therapies, artificial insemination, IVF, gametes cryopreservation, as well as fresh/frozen embryo transfer operations, in human fertility centers (deSouza et al., 2020). For those already undergoing fertility treatments, the WHO, ESHRE, and ASRM have emphasized the need to strictly follow all hygiene measures and recommendations to diminish the infection risk in those patients (deSouza et al., 2020). The global anxiety among scientific/medical associations regarding ART treatments is mainly related to the desire to avoid the exacerbating COVID-19 spread due to ART treatments or possible complications during different fertility treatments or pregnancy. However, there is a large gap in knowledge regarding COVID-19 health hazards and vertical transmission during pregnancy (Souza et al., 2020). Additionally, there is a fear that COVID-19 outbreaks could occur in ART laboratories and among medical staff (Anifandis et al., 2020). In particular, there is increased pressure on healthcare systems due to the repercussions of the COVID-19 health crisis, which exacerbates the situation (Anifandis et al., 2020). In fact, COVID-19 outbreaks could occur infertility centers or among medical staff not only due to human-to-human contact, but also due to the handling of specimens taken from patients, such as semen. This would transform fertility centers potentially to repeated sources of infection because the virus could be preserved for many years in cryopreserved semen samples of patients infected with COVID-19 (Vaiarelli et al., 2020).
Besides all of the previously mentioned obstacles making the continuation of activities at fertility centers so difficult, there are also concerns around the outcomes of different ART, for which the success rates under normal conditions are barely acceptable. For example, if COVID-19 behaves like common influenza viruses, the activation of pathogenic oxidative stress pathways can be one of the expected pathogenic pathways (Khomich et al., 2018). Elevated levels of reactive oxygen species have been identified as possible causes of male infertility (Dutta et al., 2019). These radically active molecules can adversely affect sperm cell motility and sperm DNA integrity, and thus sperm fertilizability (Hosny et al., 2020, Hashem and Gonzalez-Bulnes, 2020). Similarly, COVID-19 may affect ovum competency through the same oxidative stress pathways (Menezo et al., 2016). Given these effects in line with the negative impacts of the IVF process itself, worse IVF outcomes could easily be expected in association with COVID-19 infection (Anifandis et al., 2015).
8.1. Social impacts on reproductive health
It is believed that the COVID-19 pandemic will worsen individuals’ sex lives for multiple reasons related to physical and social factors. In fact, successful sex life and good reproductive health rely on the integration of many events, including physical, mental, emotional, and social ones. In this regard, people who have undergone quarantine and social distancing have suffered depression, irritability, poor mood, fear, guilt, and nervousness (Nimbi et al., 2018). These factors can decrease sexual desire as depression and anxiety are typically associated with low levels of desire (Nimbi et al., 2018). The fear of virus transmission via physical contact between couples is another factor negatively affecting the quality of sex life and sexual desire. The disturbances in normal sex life have pushed up the divorce rate, and promoted digital sexual relationships and self-sex practices (masturbation). All of these disruptors threaten the continuation and quality of normal sex life in couples, which may drastically affect one of the most important social entities, the family (Ibarra et al., 2020). COVID-19 has also affected the desire to bear children and the mother–child relationship due to the fear of both systematic and mental health problems of mother and child (Haruna et al., 2020). In this context, the results of a survey including 2872 pregnant women on prenatal mental health concerns during the period of COVID-19 pandemic revealed that their main concerns were: 1) COVID-19′s effects on the fetuses of infected mothers (91.0%), 2) pregnancy-associated complications when infected (74.3%), 3) the lack of certain drugs/vaccines (71.2%), 4) infections of children after birth (69.1%), 5) infections at medical centers (64.8%), and 6) inadequate antenatal support (68.4%) (MTI Ltd.).
At the same time, there is a huge pression from infertility patients overall on ART therapies. It is important to remember that the average age of couples trying to conceive is increasing each year, and the older patients arriving at fertility centers have worse ovarian reserve markers. With the extended period of the COVID-19 pandemic, as well as current recommendations to suspend ART treatments, many patients are anxious about the real possibility of compromising even further their chance of pregnancy (de Souza et al., 2020, Vaiarelli et al., 2020). Given that the success rates of IVF treatments decline at an ever-accelerating rate as the age of the female patient increases (falling by approximately 0.3% per month from the mid-thirties), it is imperative that delays to fertility treatments are minimized. Provided adequate precautions can be implemented to ensure the safety of patients attending fertility clinics, as well as that of clinical staff, it seems reasonable to consider the reintroduction of IVF treatments (at a minimum, cycles involving cryopreservation of embryos) in countries where the response to COVID-19 currently includes severe restriction or denial of patient access to fertility treatments (Stanley et al., 2020).
The impaired oxygen saturation observed in COVID-19 patients with pulmonary fibrosis could impair erectile function because oxygen is one of the substrates required for the synthesis of nitric oxide (NO) by the enzyme NO synthase, whose activity is severely reduced in hypoxia. Additionally, erectile function is a predictor of heart disease. SARS-CoV-2 can intensely affect the endothelium, heart and exacerbate underlying cardiovascular conditions. Reports of myocarditis, arrhythmias and acute cardiovascular events in COVID-19 patients have piled up and usually accompanied with erectile dysfunction. Additionally, sexual activity should be delayed until the cardiac condition has been stabilized in high-risk patients. Moreover, some drugs routinely used in COVID-19 patients such as β-blockers and antihypertensive agents can impair sexual function and might ease progression from subclinical to a clinically overt erectile dysfunction
Another aspect that should be considered for giving priority for applying any of ARTs is the health status of the couples, since couples with previous health problems, particularly hypertension and cardiovascular diseases, are highly suspected to COVID-19 infection. Available clinical data showed that approximately 15% to 30% of the COVID-19 patients are with hypertension and 2.5% to 15% are with coronary heart disease with the possibility to develop cardiovascular complications from COVID-19 infection, including arrhythmias, myocarditis, unstable coronary syndrome, and venous and arterial thromboses (Guzik et al., 2020).
9. Precautions and recommendations for safe reproductive health
Tokyo Midwives Association conducted a survey of 62 district midwife chiefs who have provided maternal and child health services in municipalities during the COVID-19 crisis. According to the data from 49 respondents, 33% of home visiting services and all mothers’ classes involving face-to-face meetings had been canceled, although midwives had begun to provide alternative services such as telephone visits, online visits, and online parenting classes (Tokyo Midwives Association, unpublished observations; as a member, the first author has permission to use the data from the association) (Haruna and Nishi, 2020).
In addition, some pregnant women have had to change their birth plans because hospitals now limit family members from attending childbirths to avoid infection. We have developed a smart phone-based cognitive-behavioral therapy (iCBT) program for pregnant women and are conducting a randomized controlled trial aimed at evaluating its effectiveness at preventing the onset of antenatal and postpartum depression.
-
1-
For sexually active couples:
-
•
Minimize the number of sexual partners you have.
-
•
Avoid kissing and having sex with partners who have symptoms of COVID-19.
-
•
Avoid sexual practices that involve the risk of passing stool into the mouth, or those that expose you or the other party to semen or urine.
-
•
Use condoms and oral rubber barriers during anal and oral sex.
-
•
Wear a mask during sex.
-
•
Wash your hands and shower before and after sexual activity, wash sex toys before and after using them, and clean the area where sexual activity occurs.
-
2-
For neonate-bearing women (Ferrazzi et al., 2020):
-
•
Use artificial feeding or start breastfeeding after a 14-day quarantine following recovery and discharge.
-
•
Follow hygiene instructions by washing hands and wearing a mask before and during newborn breastfeeding.
-
•
Use a disinfected breast pump for newborn breastfeeding to avoid direct contact.
-
3-
For infertile patients and medical staff in ART centers (Vaiarelli et al., 2020, Nishi et al., 2020):
-
•
Expand telemedicine and psychological support between patients and ART specialists.
-
•
Give priority access to new ART treatments according to urgent need (women with advanced maternal age or reduced ovarian reserve, avoiding procedures including timed sexual intercourse and IUIs for younger women, for whom the ‘time’ variable is less important).
-
•
Avoid treatment of patients highly susceptible to COVID-19 infection due to pre-existing clinical conditions, for example, renal disease, diabetes mellitus, hypertension, liver disease, heart problems, and all immunocompromised diseases, such as AIDS, cancer, and malnutrition.
-
•
Follow up the hygiene instructions and disinfection recommendations.
-
•
Adopt ART protocols that may minimize the need for frequent monitoring (ultrasound monitoring).
Minimize the social contact between different patients and different professional groups, using online medical support services.
Author contributions
NH, SA, ARA and AS: creating the main idea and writing the first draft. NH, and AS: reviewing and preparing final version of the manuscript. All the authors approved the content for publication.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number (IFKSURP-44).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nesrein M. Hashem, Email: nesreen.hashem@alexu.edu.eg.
Sameh A. Abdelnour, Email: Saelnour@zu.edu.eg.
Ahmad R. Alhimaidi, Email: ahimaidi@ksu.edu.sa.
Ayman A. Swelum, Email: aswelum@ksu.edu.sa, aymanswelum@zu.edu.eg.
References
- Abobaker A., Raba A.A. Does COVID-19 affect male fertility? World J. Urol. 2020;1–2 doi: 10.1007/s00345-020-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken R.J. COVID-19 and human spermatozoa—Potential risks for infertility and sexual transmission? Andrology. 2020 doi: 10.1111/andr.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori A., Zamarchi R., De Silvestro G., Forza G., Cavatton G., Danieli G.A. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- Anifandis G., Messini C.I., Dafopoulos K., Messinis I.E. Genes and conditions Controlling Mammalian pre- and post-implantation EmbryoDevelopment. Curr Genomics. 2015;16:32–46. doi: 10.2174/1389202916666141224205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anifandis G., Messini C.I., Daponte A., Messinis I.E. COVID-19 and fertility: A virtual reality. Reprod Biomed Online. 2020;41:157–159. doi: 10.1016/j.rbmo.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L., Merrill D.C., Neves L.A., Diz D.I., Corthorn J., Valdes G. The uterine placental bed renin-angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150:4316–4325. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L., Merrill D.C., Neves L.A., Stovall K., Gallagher P.E., Diz D.I. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension. 2008;51:1066–1072. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafat S.M.Y., Alradie-Mohamed A., Kar S.K., Sharma P., Kabir R. Does COVID-19 pandemic affect sexual behaviour? A cross-sectional, cross-national online survey. Psychiatry Res. 2020;289 doi: 10.1016/j.psychres.2020.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi-Yazdi M., Nadjarzadeh A., Khosravi-Boroujeni H., Salehi-Abargouei A. The effect of Vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2017;49:174–179. doi: 10.1055/s-0043-103573. [DOI] [PubMed] [Google Scholar]
- Barreta M.H., Gasperin B.G., Ferreira R., Rovani M., Pereira G.R., Bohrer R.C. The components of the angiotensin-(1–7) system are differentially expressed during follicular wave in cattle. J Renin Angiotensin Aldosterone Syst. 2015;16:275–283. doi: 10.1177/1470320313491996. [DOI] [PubMed] [Google Scholar]
- Brosnihan K.B., Bharadwaj M.S., Yamaleyeva L.M., Neves L.A. Decidualized pseudopregnant rat uterus shows marked reduction in Ang II and Ang-(1–7) levels. Placenta. 2012;33:17–23. doi: 10.1016/j.placenta.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth M., McClellan B., Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–1225. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- Castillo J., Jodar M., Oliva R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum Reprod Update. 2018;24:535–555. doi: 10.1093/humupd/dmy017. [DOI] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine verticaltransmission potential of COVID-19 infectionin nine pregnant women: a retrospectivereview of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, ... Yip CC. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: Systematic review and meta-analysis. Gastroenterology (2020) S0016–5085: 30448. https ://doi.org/10.1053/j.gastr o.2020.03.065. [DOI] [PMC free article] [PubMed]
- Costa A.P., Fagundes-Moura C.R., Pereira V.M., Silva L.F., Vieira M.A., Santos R.A. Angiotensin-(1–7): a novel peptide in the ovary. Endocrinology. 2003;144:1942–1948. doi: 10.1210/en.2002-220787. [DOI] [PubMed] [Google Scholar]
- Cui P., Chen Z., Wang T., Dai J., Zhang J., Ding T. Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: A descriptive study in Wuhan. China. medRxiv. 2020 [Google Scholar]
- de Souza M.D.C.B., Nakagawa H., Taitson P.F., Cordts E.B., Antunes R.A. Management of ART and COVID-19: infertility in times of pandemic. What now? JBRA Assist Reprod. 2020;24:231–232. doi: 10.5935/1518-0557.20200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARSCoV-2 Froman infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S., Majzoub A., Agarwal A. Oxidative stress and sperm function:expressed in the human ovary. Fertil Steril. 2019;95:176–181. [Google Scholar]
- Fan C, Lei D, Fang C, LI C, Wang M, Liu Y et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry?Clin Infect Dis (2020). pii: ciaa226. https://doi.org/.org/10.1093/cid/ciaa226.
- Fang F., Ni K., Cai Y., Shang J., Zhang X., Xiong C. Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2017;26:53–60. doi: 10.1016/j.ctcp.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S. B.J.O.G; Int J Obstet Gynaecol: 2020. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadhanfar E., Alsalem A., Al-Kandari S., Naser J., Babiker F., Al-Bader The role of ACE2, angiotensin-(1–7) and Mas1 receptor axis in glucocorticoid-induced intrauterine growth restriction. Reprod Biol Endocrinol. 2017;15:1–9. doi: 10.1186/s12958-017-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden T.N., Simmons R.A. Maternal and neonatal response to COVID-19. Am J Physiol Endocrinol Metab. 2020;319:E315–E319. doi: 10.1152/ajpendo.00287.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren A., McCoy J., Wambier C.G., Vano-Galvan S., Shapiro J., Dhurat R. What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy. Dermatol Ther. 2020;33 doi: 10.1111/dth.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L., Wang H., Sun X., Ahmad S., Ferrario C.M. Is sex a determinant of COVID-19 infection? truth or myth? Current Hypertension Reports. 2020;22(9):1–12. doi: 10.1007/s11906-020-01073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X. The adult human testis transcriptional cell atlas. Cell Res. 2018;28:1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna M., Nishi D. Perinatal mental health and COVID-19 in Japan. Psychiatry Clin Neurosci. 2020 doi: 10.1111/pcn.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem N.M., Gonzalez-Bulnes A. State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals (Basel) 2020;10:840. doi: 10.3390/ani10050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem N.M., Gonzalez-Bulnes A., Rodriguez-Morales A.J. Animal welfare and livestock supply chain sustainability under COVID-19 outbreak: an Overeview. Front Vet Sci. 2020 doi: 10.3389/fvets.2020.582528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K.G., Acosta T.J., Tetsuka M., Berisha B., Matsui M., Schams D. Involvement of angiopoietin-tie system in bovine follicular development and atresia: messenger RNA expression in theca interna and effect on steroid secretion. Biol Reprod. 2003;69:2078–2084. doi: 10.1095/biolreprod.103.017152. [DOI] [PubMed] [Google Scholar]
- Hosny N.S., Hashem N.M., Morsy A.S., Abo-elezz Z.R. Effects of organic selenium on the physiological response, blood metabolites, redox status, semen quality, and fertility of rabbit bucks kept under natural heat stress conditions. Front Vet Sci. 2020;7:290. doi: 10.3389/fvets.2020.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra FP, Mehrad M, Di Mauro MD, Godoy MFP, Cruz EG, Nilforoushzadeh MA et al. Impact of the COVID-19 pandemic on the sexual behavior of the population. The vision of the east and the west. Int Braz J Urol (2020) 46:104-12. [DOI] [PMC free article] [PubMed]
- Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joguet G., Mansuy J.-M., Matusali G. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis. 2017;17:1200–1208. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]
- Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox biology of respiratory viral infections. Viruses. 2018;10:392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Kloc M., Ghobrial R.M., Kubiak J.Z. The Role of Genetic Sex and Mitochondria in Response to COVID-19 Infection. Int Arch Allergy Immunol. 2020;181:629–634. doi: 10.1159/000508560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I., Karteris E., Robbins T., Chatha K., Drenos F., Randeva H.S. Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked female patient population at potentially higher risk during the COVID-19 pandemic. B.M.C. Med. 2020;18(220) doi: 10.1186/s12916-020-01697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vignera S., Cannarella R., Condorelli R.A., Torre F., Aversa A., Calogero A.E. Sex-specific SARS-CoV-2 mortality: among hormone-modulated ACE2expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci. 2020;21:E2948. doi: 10.3390/ijms21082948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal M.C., Pinheiro S.V., Ferreira A.J., Santos R.A., Bordoni L.S., Alenina N., Bader M., França L.R. The role of angiotensin-(1–7) receptor Mas in spermatogenesis in mice and rats. J Anat. 2009;214:736–743. doi: 10.1111/j.1469-7580.2009.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical character-istics and results of semen tests among men with coronavirus disease 2019. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. https ://doi.org/10.1001/jaman etwor kopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiao X., Zhang J., Zafar M.I., Wu C., Long Y. Impaired spermatogenesis in COVID-19 patients. The Lancet. 2020;8 doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.F., Ahmed A. Expression of angiotensin II and its receptor subtypes in endometrial hyperplasia: a possible role in dysfunctional menstruation. Lab Invest. 1996;75:137–145. [PubMed] [Google Scholar]
- Lin T., Hu J., Wang D., Stocco D.M. Interferon-γ inhibits the steroidogenic acute regulatory protein messenger ribonucleic acid expression and protein levels in primary cultures of rat Leydig cells. Endocrinology. 1998;139:2217–2222. doi: 10.1210/endo.139.5.6006. [DOI] [PubMed] [Google Scholar]
- Liu T., Hu J., Xiao J., He G., Kang M., Rong Z. Time-varying transmission dynamics of Novel Coronavirus Pneumonia in China. BioRxiv. 2020 [Google Scholar]
- Liu W., Han R., Wu H., Han D. Viral threat to male fertility. Andrologia. 2018;50 doi: 10.1111/and.13140. [DOI] [PubMed] [Google Scholar]
- Loveland K.L., Klein B., Pueschl D., Indumathy S., Bergmann M., Loveland B.E., Hedger M.P., Schuppe H.C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front Endocrinol (Lausanne) 2017;8:307. doi: 10.3389/fendo.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Xie W., Li D. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2020:1–7. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q., Wu W., Liao Z. Viral pathogens hitchhike with insect sperm for paternal transmission. Nat Commun. 2019;10:955. doi: 10.1038/s41467-019-08860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Klein SL, and Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology (2020) 161:bqaa127. [DOI] [PMC free article] [PubMed]
- Menezo Y.J., Silvestris E., Dale B., Elder K. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online. 2016;33:668–683. doi: 10.1016/j.rbmo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- MTI Ltd. Shingatakoronauirusukansensyonikansuru “LunaLuna” dokujicyousa. 2020 (in Japanese). https://prtimes.jp/main/html/rd/p/000000688.000002943.html [Cited 1 June 2020].
- Navarra A, Albani E, Castellano S, Arruzzolo L, Levi-Setti PE. Coronavirus Disease-19 Infection: Implications on Male Fertility and Reproduction. Front Physiol (2020) | https://doi.org/10.3389/fphys.2020.574761 [DOI] [PMC free article] [PubMed]
- Mo Y, Deng L, Zhang L, Lang Q, Liao C, Wang N, Qin M, Huang H. Work stress among Chinese nurses to support Wuhan in fighting against COVID-19 epidemic. J Nurs Manag. 2020;28(5):1002–1009. doi: 10.1111/jonm.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimbi F.M., Tripodi F., Rossi R., Simonelli C. Expandingthe analysis of psychosocial factors of sexual desire inMen. J Sex Med. 2018;15:230–244. doi: 10.1016/j.jsxm.2017.11.227. [DOI] [PubMed] [Google Scholar]
- Nishi D., Imamura K., Watanabe K., Obikane E., Sasaki N., Yasuma N. Internet-based cognitive–behavioural therapy for prevention of depression during pregnancy and in the post partum (iPDP): a protocol for a large-scale randomized controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O., Laster J., Loyter A. Fusion of enveloped viruses with sperm cells: interaction of Sendai, influenza, and Semliki Forest viruses with bull spermatozoa. Exp Cell Res. 1993;206(11–15):27. doi: 10.1006/excr.1993.1114. [DOI] [PubMed] [Google Scholar]
- Obermüller N., Gentili M., Gauer S., Gretz N., Weigel M., Geiger H. Immunohistochemical and mRNA localization of the angiotensin II receptor subtype 2 (AT2) in follicular granulosa cells of the rat ovary. J Histochem Cytochem. 2004;52:545–548. doi: 10.1177/002215540405200413. [DOI] [PubMed] [Google Scholar]
- Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020;43:1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. ;: . 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P-P, Zhan Q-T, Le F, Zheng Y-M, Jin F. Angiotensinconverting enzymes play a dominant role in fertility. Int J Mol Sci. 2013;14:21071–21086. doi: 10.3390/ijms141021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O., Antonelli G., Lenzi A., Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive rino-pharyngeal swab. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira V.M., Reis F.M., Santos R.A., Cassali G.D., Santos S.H., Honorato-Sampaio K. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod Sci. 2009;16:1165–1174. doi: 10.1177/1933719109343309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique-Regi R, Romero R, Tarca AL, Luca F, Xu Y, Alazizi A et al. Does the human placenta express the canonicalcell entry mediators for SARS-CoV-2? Elife (2020). https://doi.org/.org/10.1101/2020.05.18.101485. [DOI] [PMC free article] [PubMed]
- Pringle KG, Tadros MA, Callister RJ, and Lumbers ER. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis?Placenta (2011) 32:956–62. https://doi.org/.org/10.1016/j.placenta.2011.09.020. [DOI] [PubMed]
- Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus sisease 2019 (COVID19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. 10.1016/j.ajog.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, A.B., Araújo F.C., Pereira V.M. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Hist. 2010;41:75–80. doi: 10.1007/s10735-010-9264-8. [DOI] [PubMed] [Google Scholar]
- Reis F.M., Bouissou D.R., Pereira V.M., Camargos A.F., dos Reis A.M., Santos R.A. Angiotensin-(1–7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95:176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- Reis G.V., Gontijo N.A., Rodrigues K.F., Alves M.T., Ferreira C.N., Gomes K.B. Vitamin D receptor polymorphisms and the polycystic ovary syndrome: a systematic review. J Obstet Gynaecol Res. 2017;43:436–446. doi: 10.1111/jog.13250. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? The Lancet. 2020;395:10231. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Bonilla-Aldana DK, Tiwari R, Sah R, Rabaan AA, and Dhama K. COVID-19, an emerging coronavirus infection: current scenario and recent developments-an overview. J Pure Appl Microbiol (2020) 14:05–12.https://doi.org/.org/10.22207/JPAM.14.1.02.
- Sansone A., Rastrelli G., Cignarelli A., de Rocco P.M., Condorelli R.A., Giannetta E. Effect of treatment with testosterone on endothelial function in hypogonadal men: a systematic review and meta-analysis. Int J Impot Res. 2019 doi: 10.1038/s41443-019-0163-6. [DOI] [PubMed] [Google Scholar]
- Segars J, Katler Q, McQueen DB, Kotlyar A, Glenn T, Knight Z et al. Prior and novel coronaviruses, COVID-19, and human reproduction: what is known? Fertil Steril (2020) 113:1140–9’. [DOI] [PMC free article] [PubMed]
- Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, and Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol (2020) 154:23–32.https://doi.org/.org/10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed]
- Shen Q., Xiao X., Aierken A. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection [published online ahead of print, 2020 Jun 28] J Cell Mol Med. 2020;24(9472–9477) doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E., Powers R.W., Rajakumar A., von Versen-Höynck F., Gallaher M.J., Lykins D.L. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am J Physiol Endocrinol Metab. 2006;291:E1009–E1016. doi: 10.1152/ajpendo.00134.2006. [DOI] [PubMed] [Google Scholar]
- Singh M, Bansal V, Feschotte C. A single-cell RNA expression map of human coronavirus entry factors. BioRxiv (2020) ;2020 [DOI] [PMC free article] [PubMed]
- Siston A.M., Rasmussen S.A., Honein M.A., Fry A.M., Seib K., Callaghan W.M. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proceedings of the National Academy of Sciences of the United States of America (2020) 117:11875–11877. https ://doi.org/10.1073/pnas.20068 74117 [DOI] [PMC free article] [PubMed]
- Song C., Wang Y., Li W., Hu B., Chen G., Xia P., Wang W., Li C., Diao F., Hu Z., Yang X., Yao B., Liu Y. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients†. Biol Reprod. 2020 Jun 23;103(1):4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K.E., Thomas E., Leaver M., Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33–43. doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino N., Suzuki T., Sakata A., Miwa I., Asada H., Taketani T. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2005;90:6141–6148. doi: 10.1210/jc.2005-0643. [DOI] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M et al.َpez C, Maatz H, Reichart D, Sampaziotis F, Worlock KB,Yoshida M, Barnes JL; HCA Lung Biological Network. SARS-CoV-2entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26: 681–687, 2020. doi:10.1038/s41591020-0868-6. [DOI] [PMC free article] [PubMed]
- Tang K., Gaoshan J., Ahons B., Moazzam Ali M., Bonet Edna Kara. Caron Kim, anna Thorsonand soe soe. ThwinSexual and reproductive health (SRH): a keyissue in the emergency response to the coronavirus disease (COVID- 19) outbreakReproductive. Health. 2020;17(59) doi: 10.1186/s12978-020-0900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. Recommendations from the international evidence-based guideline for theassessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonellotto dos Santos J., Ferreira R., Gasperin B.G., Siqueira L.C., de Oliveira J.F., Santos R.A. Molecular characterization and regulation of the angiotensin-converting enzyme type 2/angiotensin-(1–7)/MAS receptor axis during the ovulation process in cattle. J Renin Angiotensin Aldosterone Syst. 2012;13:91–98. doi: 10.1177/1470320311417273. [DOI] [PubMed] [Google Scholar]
- Vaiarelli A., Bulletti C., Cimadomo D., Borini A., Alviggi C., Ajossa S. COVID-19 and ART: the view of the Italian Society of Fertility and Sterility and Reproductive Medicine. Reprod Biomed Online. 2020;40:755–759. doi: 10.1016/j.rbmo.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés G., Corthorn J., Bharadwaj M.S., Joyner J., Schneider D., Brosnihan K.B. Utero-placental expression of angiotensin-(1–7) and ACE2 inthe pregnant guinea-pig. Reprod Biol Endocrinol. 2013;11:5. doi: 10.1186/1477-7827-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés G., Neves L.A., Anton L., Corthorn J., Chacَn C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normaland pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Valdivia A., Cortés L., Beitia M. Role of Angiotensin-(1–7) via MAS receptor in human sperm motility and acrosome reaction. Reproduction. 2020;159:241–249. doi: 10.1530/REP-19-0274. [DOI] [PubMed] [Google Scholar]
- Vaz-Silva J., Carneiro M.M., Ferreira M.C., Pinheiro S.V., Silva D.A., Silva-Filho A.L. The vasoactive peptide angiotensin-(1–7), its receptor Mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod Sci. 2009;16:247–256. doi: 10.1177/1933719108327593. [DOI] [PubMed] [Google Scholar]
- Vaz-Silva J., Tavares R.L., Ferreira M.C., Honorato-Sampaio K., Cavallo I.K., Santos R.A. Tissue specific localization of angiotensin-(1–7) and its receptor Mas in the uterus of ovariectomized rats. J Mol Histol. 2012;43:597–602. doi: 10.1007/s10735-012-9427-x. [DOI] [PubMed] [Google Scholar]
- Verma S., Saksena S., Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis. Biol Reprod. 2020;103:449–451. doi: 10.1093/biolre/ioaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti A.J., Vauloup-Fellous C., Prevot S. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhou X., Zhang T., Wang Z. The need for urogenital tract monitoring in COVID-19. Nat Rev Urol. 2020;17:314–315. doi: 10.1038/s41585-020-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wan J., Ling X., Liu M., Zhou T. The human sperm proteome 2.0: An integrated resource for studying sperm functions at the level of posttranslational modification. Proteomics. 2016;16:2597–2601. doi: 10.1002/pmic.201600233. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia. Leydig and Sertoli Cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020). Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. Retrieved April 3, 2020, from https://www.who.int/news-room/ commentaries/detail/modes-of-transmission-of-virus-causing-covid19-implications-for-ipc-precaution-recommendations
- Wu Y., Liu C., Dong L., Zhang C., Chen Y., Liu J., Dennis C.L. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG: An International Journal of Obstetrics & Gynaecology. 2020;127(9):1109–1115. doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., Peh S., Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Yang M., Guo H., Yang L., Wu J., Li R. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- Yang M., Chen S., Huang B., Zhong J.M., Su H., Chen Y.J. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. European Urology Focus. 2020;6:1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis J.S., Abassi Z., Skorecki K. Is there an impact of the COVID-19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 2020;318:E878–E880. doi: 10.1152/ajpendo.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Yan L., Wang N., Yang S., Wang L., Tang Y., Wang F. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clinical Infectious Diseases. 2020;71(793–798) doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel B., Ozgor F. Effect of the COVID-19 pandemic on female sexual behavior. Int J Gynaecol Obstet. 2020;150:98–102. doi: 10.1002/ijgo.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARSCoV2 in 33 neonates born to mothers with COVID19 in Wuhan. China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirusof probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]