Abstract
Objective
To describe the demographics, clinical features, and test results of children referred from their primary provider for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the community setting.
Study design
Retrospective cross-sectional study of children ≤22 years of age who were tested for SARS-CoV-2 at a community-based specimen collection site in Washington, DC, affiliated with a large children's hospital between March 21 and May 16, 2020.
Results
Of the 1445 patients tested at the specimen collection site for SARS-CoV-2 virus, 408 (28.2%) had a positive polymerase chain reaction test. The daily positivity rate increased over the study period, from 5.4% during the first week to a peak of 47.4% (Ptrend < .001). Patients with fever (aOR, 1.7; 95% CI, 1.3-2.3) or cough (aOR, 1.4; 95% CI, 1.1-1.9) and those with known contact with someone with confirmed SARS-CoV-2 infection (aOR, 1.6; 95% CI, 1.0-2.4.) were more likely have a positive test, but these features were not highly discriminating.
Conclusions
In this cohort of mildly symptomatic or well children and adolescents referred to a community drive-through/walk-up SARS-CoV-2 testing site because of risk of exposure or clinical illness, 1 in 4 patients had a positive test. Children and young adults represent a considerable burden of SARS-CoV-2 infection. Assessment of their role in transmission is essential to implementing appropriate control measures.
Keywords: pediatric, mildly symptomatic, asymptomatic, children, COVID-19, SARS-CoV-2
Abbreviations: COVID-19, Novel coronavirus disease-2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2
As of May 30, 2020, 1.3 million cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection had been reported in the US and more than 69 000 (5%) are among youth.1 In the US, 22% of the population is composed of youth <18 years old. Experience from China and Europe suggests that children of all ages are susceptible to SARS-CoV-2, but the disease tends to be clinically milder compared with adults, with as many as 90% infected children being asymptomatic or having only mild or moderate symptoms.2, 3, 4 Recent US data confirm this finding, but also include findings of a subset of patients with serious illness resulting in hospitalization, documenting that serious illness does occur in the pediatric population, possibly at higher rates than previously reported.5 , 6
A common challenge during the novel coronavirus disease-2019 (COVID-19) pandemic has been inadequate diagnostic testing capacity, limited personal protective equipment, and the need for social distancing in health care settings.7 Drive-through/walk-up specimen collection sites for SARS-CoV-2 offer a community-based solution to these challenges. Regional variation in testing availability limits the ability to describe the referral patterns and clinical presentation of pediatric patients in the community setting. However, as the US reopens schools and childcare centers, information about the community spread of SARS-CoV-2 among children has attracted great attention to guide recovery planning.
Drive-through/walk-up testing sites outside of a traditional acute care setting have emerged around the world to meet the need for testing mildly ill or asymptomatic individuals.8 , 9 Community-based testing sites have the advantages of minimizing exposure risk to other patients and healthcare workers, preserving personal protective equipment, centralizing specimen collection services, mitigating acute care site overcrowding, and informing public health authorities of the community's disease burden. Responding to the needs of patients served by an affiliated clinically integrated network of pediatric practices in the Washington, DC, metropolitan region, the COVID response team at a free-standing pediatric hospital established an exclusively pediatric SARS-CoV-2 testing site on March 21, 2020. The primary objective of this study was to describe the features of children tested at this site who did not require acute medical care. The secondary objective was to compare demographic and clinical differences between patients who tested positive and negative for SARS-CoV-2.
Methods
This was a cross-sectional study of children tested for SARS-CoV-2 at an exclusively pediatric drive-through/walk-up urban specimen collection site affiliated with a large, tertiary care children's hospital in Washington, DC, from March 21 to May 16, 2020. Patients were referred by community pediatricians through an online registration and referral process. Self-referral was not permitted. Referring clinicians included members of an affiliated clinically integrated network of >500 regional pediatricians. Pediatricians were advised to refer patients between the ages of 0 and 22 years if they reported mild symptoms or met the following criteria: patients who were at high risk for serious infection, patients who lived with high-risk household members, or patients who lived with household members whose work status would be impacted by the presence of infection. The site was located within 1 mile of the hospital at an outdoor parking lot of a local university and was operational 2-3 days per week. Patients were seen in single file and remained in their vehicle, or, if ambulatory, ≥6 feet apart from other patients. All specimens were collected via nasopharyngeal or oropharyngeal swab by trained healthcare workers and sent to an offsite commercial laboratory (Quest Diagnostics, Inc) for Emergency Use Authorization-approved real-time reverse transcription polymerase chain reaction testing. Results were returned to the referring provider. The hospital's institutional review board approved this study.
Data Collection
Demographic and clinical data were extracted from the electronic referral forms and merged with laboratory results from the electronic medical record system. Collected data included age, sex, race/ethnicity, and home address. Consistent with other studies, race/ethnicity was categorized as non-Hispanic Black, non-Hispanic White, Hispanic, and other.10 Age was organized into the following categories: <1, 1-4, 5-11, 12-17, and 18-22 years of age. Home address was used to measure the distance from the testing site. The reason for referral was categorized as high-risk patient for exposure or infection, household member with a high risk for exposure or infection, need to know status for work or contact tracing, and known exposure. Reasons for referral were not mutually exclusive. Symptoms were classified into the following nonmutually exclusive categories: none, upper respiratory tract symptoms (eg, sore throat or nasal congestion), cough, lower respiratory tract symptoms (eg, difficulty breathing), gastrointestinal symptoms (eg, vomiting or diarrhea), neurologic symptoms (eg, headache), and systemic symptoms (eg, fever). The presence of symptoms was analyzed as a binary variable (yes/no).
Statistical Analyses
We used standard descriptive statistics to summarize the study population. We performed bivariable logistic regression analyses to calculate unadjusted and aOR with 95% CI to identify demographic and clinical characteristics associated with SARS-CoV-2 infection. We also performed multivariable logistic regression that was a priori adjusted for age, race/ethnicity, sex, and distance from testing site. We calculated SARS-CoV-2 positivity rates and assessed temporal trends in positivity rates over the study period using p-trend analyses. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Geospatial analysis was conducted using ArcGIS Pro (Esri, Redlands, California). Home addresses were geocoded to create a point layer with all patients tested and patients tested positive. Patients were excluded from the geospatial analysis if they had invalid addresses (n = 12). The point layers were spatially joined with a zip code polygon layer to develop a map with pie charts to view the spatial distribution of testing and positivity by zip code.
Results
Testing Numbers and Burden of Infection
During the study period, 1445 patients were referred to the testing site for specimen collection. An average of 79 patients (IQR, 53.0-104.5 patients) were tested each day. There were 408 patients who tested positive (28.2%, 95% CI, 18.2-23.3.) The average daily rate of positivity ranged from 5.4% in the first week of testing site opening (March 21-29) to a peak of 47.4% on May 5. The rate of positivity for the last week of the study period (May 9-16) was 37.8% (P trend < .001) (Figure 1 ).
Figure 1.
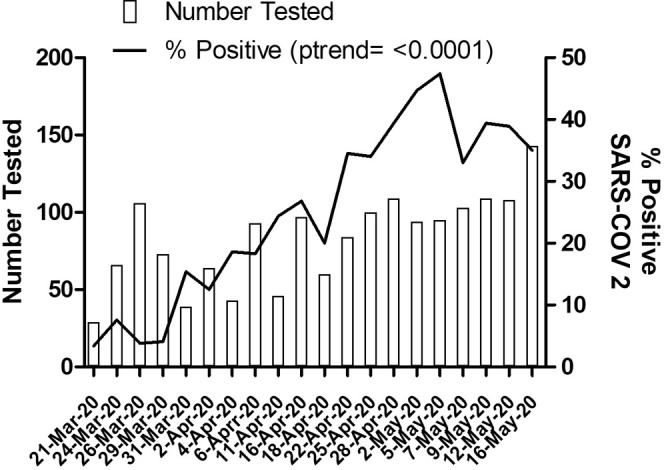
Trends in testing and positivity rates. Average number of daily SARS-CoV-2 specimen collections and positive test rates.
Characteristics of Tested Patients
The median age of all the patients tested was 8 years (IQR, 3-14 years) and 34.7% of patients were Hispanic ethnicity, followed by non-Hispanic Black (28.3%) and non-Hispanic White (16.8%) (Table I ).
Table I.
Selected patient demographics of patients referred for SARS-COV-2 testing
| Characteristics | Total | Result status |
Odds of positive test |
||
|---|---|---|---|---|---|
| Negative (n = 1037; 71.8%) | Positive (n = 408; 28.2%) | OR (95% CI) | aOR (95% CI)∗ | ||
| Age, years | |||||
| <1 | 119 (8.2%) | 83 (8.0) | 36 (8.8) | Referent | Referent |
| 1-4 | 383 (26.5) | 311 (30.0) | 72 (17.6) | 0.5 (0.3-0.9) | 0.5 (0.3-0.8) |
| 5-11 | 428 (29.6) | 292 (28.2) | 136 (33.3) | 1.1 (0.7-1.7) | 0.8 (0.5-1.3) |
| 12-17 | 344 (23.8) | 224 (21.6) | 120 (29.4) | 1.2 (0.8-1.9) | 1.0 (0.6-1.6) |
| 18-22 | 171 (11.8) | 127 (12.3) | 44 (10.8) | 0.8 (0.5-1.3) | 0.7 (0.4-1.2) |
| Sex | |||||
| Female | 697 (48.2) | 496 (47.8) | 201 (49.3) | Referent | Referent |
| Male | 733 (50.7) | 539 (52.0) | 194 (47.6) | 0.9 (0.7-1.1) | 0.9 (0.7-1.2) |
| Not documented | 15 (1.0) | 2 (0.2) | 13 (3.2) | -- | -- |
| Race/ethnicity | |||||
| Non-Hispanic White | 243 (16.8) | 225 (21.7) | 18 (4.4) | Referent | Referent |
| Non-Hispanic Black | 409 (28.3) | 305 (29.4) | 104 (25.5) | 4.3 (2.5-7.2) | 4.1 (2.4-7.0) |
| Hispanic | 501 (34.7) | 268 (25.8) | 233 (57.1) | 10.9 (6.5-18.1) | 10.3 (6.2-17.3) |
| Other | 173 (12.0) | 147 (14.2) | 26 (6.4) | 2.2 (1.2-4.2) | 2.3 (1.2-4.4) |
| Unknown/missing | 119 (8.2) | 92 (8.9) | 27 (6.6) | -- | -- |
| Distance from testing site-miles | 7.3 (4.3-12.9) | 7.0 (2.0-14.0) | 10 (4.0-14.0) | 0.9 (0.9-1.0) | 0.9 (0.9-1.0) |
| Referral visit type | |||||
| Office | 110 (7.6) | 75 (7.2) | 35 (8.6) | Referent | Referent |
| Telemedicine | 640 (44.3) | 455 (43.9) | 185 (45.3) | 0.9 (0.6-1.3) | -- |
| Phone | 223 (15.4) | 129 (12.4) | 94 (23.0) | 1.6 (0.9-2.5) | -- |
| Missing | 472 (32.7) | 378 (36.5) | 94 (23.0) | -- | -- |
Values are number (%) or median (IQR) unless otherwise noted.
Adjusted for age, race/ethnicity, sex, and distance from specimen collection site.
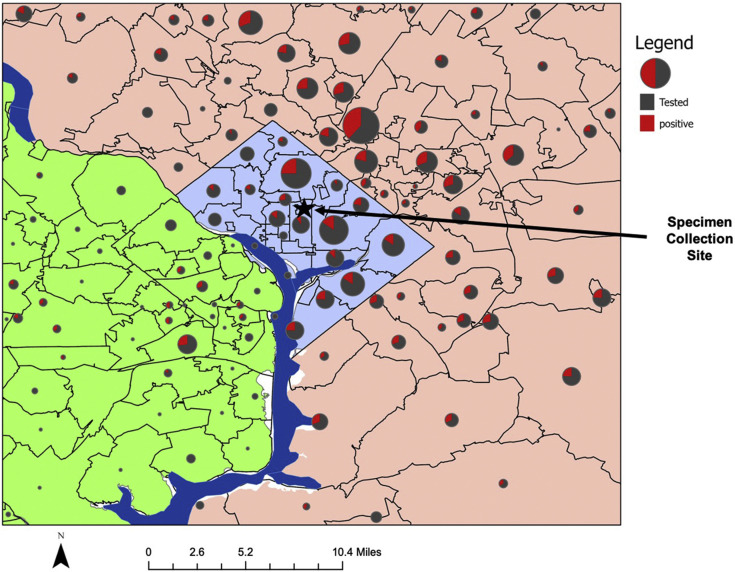
Patients were referred to the testing site using telemedicine (44.3%), office visits (15.4%), and telephone calls (7.6%). The most common reasons were a high-risk family member (35%) or need to know for work (30%) (Figure 2 ). Of those tested, 83.7% (n = 1210) had symptoms documented by the referring provider. The most common symptoms reported were lower respiratory complaints (41.6%) and fever (35%) (Table II ). The median distance from the patients’ homes to the specimen collection site was 7.3 miles (IQR, 4.3-12.9 miles); the farthest distance traveled was 73.6 miles (Figure 3; available at www.jpeds.com).
Figure 2.
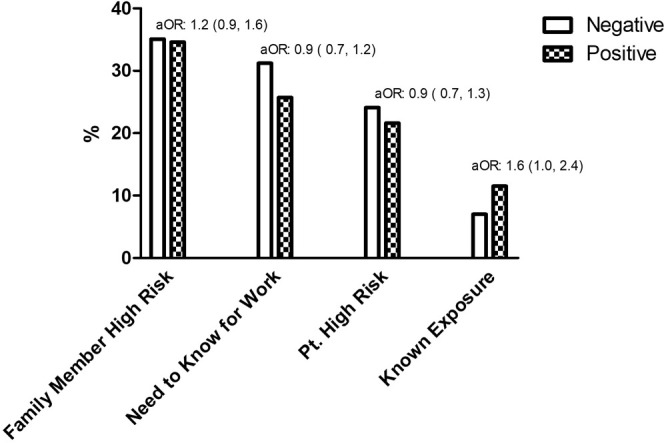
Referral reasons for SARS-CoV-2 testing. Frequency of referral reasons for SARS-CoV-2 testing.
Table II.
Reported symptoms among patients referred for SARS-COV-2 testing (n = 1210)
| Symptoms∗ | No. (%) | Result status |
Odds of positive test |
||
|---|---|---|---|---|---|
| Negative (n = 832; 68.8%) | Positive (n = 378; 31.2%) | OR (95% CI) | aOR (95% CI)† | ||
| None | 411 (34.0) | 288 (34.6) | 123 (32.5) | 0.9 (0.7-1.2) | 0.8 (0.6-1.0) |
| Fever | 423 (35.0) | 276 (33.2) | 147 (38.9) | 1.3 (1.0-1.7) | 1.7 (1.3-2.3) |
| Neurologic | 195 (16.1) | 124 (14.5) | 71 (18.8) | 1.3 (1.0-1.8) | 1.2 (0.9-1.8) |
| Upper respiratory tract symptoms | 385 (32.0) | 266 (32.0) | 119 (31.5) | 1.0 (0.9-1.3) | 1.1 (0.8-1.4) |
| Cough | 487 (40.3) | 213 (37.5) | 175 (46.3) | 1.4 (1.1-1.8) | 1.4 (1.1-1.9) |
| Lower respiratory symptoms | 67 (5.5) | 54 (6.5) | 13 (3.4) | 0.5 (0.3-0.9) | 0.5 (0.3-1.0) |
| Gastrointestinal symptoms | 150 (12.4) | 115 (13.8) | 35 (9.3) | 0.6 (0.4-0.9) | 0.8 (0.5-1.2) |
Bold represents a P value of < .05. Values are number (%) unless otherwise indicated.
Not mutually exclusive.
Adjusted for age, race/ethnicity, sex, and distance from specimen collection site.
Figure 3.
Geographic distribution of patients referred to specimen collection site. Geographic distribution of patients referred to testing site across 3 distinct regions: Washington DC, Maryland, and Virginia. The star and the arrow show the location of the specimen collection site.
Characteristics of Patients who were SARS-CoV-2-Positive
We found no notable sex or age differences among those with confirmed infection. Compared with non-Hispanic White children and after adjustments for age, sex, and distance of residence from specimen collection site, minority children had a higher likelihood of infection (Hispanic, aOR, 10.3; 95% CI, 6.2-17.3; non-Hispanic Black, aOR, 4.1; 95% CI, 2.4-7.0) (Table I).
Patients who self-reported a known exposure to COVID-19 were more likely to test positive compared with those who did not report a known exposure (aOR, 1.6; 95% CI, 1.0-2.4). Other reasons for referral (need to know for work, high-risk patient for exposure or infection, household member with high risk for exposure or infection) were not associated with positivity. Patients with a cough (aOR, 1.4; 95% CI, 1.1-1.9) or fever (aOR, 1.7; 95% CI, 1.3-2.3) were more likely to have a positive test than patients without those symptoms. Neurologic, upper respiratory tract symptoms, lower respiratory tract symptoms and gastrointestinal symptoms were not associated with testing positive in our population (Table II).
Discussion
This study describes the clinical and demographic characteristics of a large sample of children referred to a community-based SARS-CoV-2 drive-through/walk-up testing site in Washington, DC, because of the risk of exposure or infection or both. The overall positivity rate was nearly 30%; rates increased throughout the study period from approximately 5% to almost 50% at the peak on May 5, 2020. The daily positivity rates throughout the study period followed a trend similar to the overall population's SARS-CoV-2 rates for the Washington, DC, and the surrounding regions, suggesting that children have a similar prevalence of viral infection compared with adults with risk factors for exposure, despite different rates of illness severity in children.11
Patients with COVID-19 exposure and symptoms were more likely to have a positive test than patients without symptoms. This finding supports the need for contact tracing for symptomatic cases and for testing as important tools in detecting and containing community spread. Although most patients were referred because they lived with a family member with a high risk for exposure or infection, this factor was not associated with positive test results.
To alleviate the risk of unintentional viral transmission between patients and healthcare providers, physicians have rapidly adopted telemedicine during the pandemic.12 In line with this recent transition, most referrals to the testing site were after telemedicine visits. The drive-through site allows pediatricians to avoid having to bring potentially infectious patients into their offices that may not be equipped with the personal protective equipment required to handle test collection safely.
This study had several limitations. This was a retrospective study reliant on the accuracy of the data collected at the time of physician referral and the electronic health record. Approximately one-third of patients tested were young children (≤5 years of age); therefore, the number and type of reported symptoms may have been underestimated. The small number of patients <1 year of age limits our ability to confidently comment on the symptomatology and severity of illness in infants with positive SARS-CoV-2 tests. Although this testing site was a unique resource for children early in the pandemic, over time, additional venues opened; therefore, these data may not represent the entire spectrum of children undergoing testing who have mild illness or a high risk of exposure.
The impact of SARS-CoV-2 is broad and impacts planning for children, especially as schools and childcare centers reopen. Understanding the community prevalence of infection in the pediatric population may also have implications for individuals who reside or work with children. A determination of the transmission potential of these mildly symptomatic or well children and young adults is important for guiding the development of measures to control the ongoing pandemic.
Data Statement
Data sharing statement available at www.jpeds.com.
Acknowledgments
We thank the Children's National Hospital and George Washington University medical student volunteers that worked at the testing site. We also thank members of the Children's National Hospital COVID-19 Task Force under the leadership of Dr Kurt Newman that supported the planning and execution of the testing site. We are also grateful to Pat McGuire (President) and Ann Pauley (Vice President for Institutional Advancement/Media Relations) of Trinity Washington University who provided the location and ground support so that so many children in our region could have access to testing. Last, we wish to express gratitude on behalf of the children and families served by our testing site to our philanthropic donors who made the initiative possible.
Footnotes
The authors declare no conflicts.
Supplementary Data
Appendix
References
- 1.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Zhou Q., Wang C., Shi Q., Lu S., Ma Y., et al. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med. 2020;8:620. doi: 10.21037/atm-20-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagnoli R., Votto M., Licari A., Brambilla I., Bruno R., Perlini S., et al. Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 5.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J., et al. Severe COVID-19 in children and young adults in the Washington, DC Metropolitan region. J Pediatr. 2020;233:199–203.e1. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P.N., Finger L., Schieffelin J.S., Zerr D.M., Hametz P.A. Responses of three urban U.S. Children's Hospitals to COVID-19: Seattle, New York and New Orleans. Paediatr Respir Rev. 2020;35:15–19. doi: 10.1016/j.prrv.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon K.T., Ko J.H., Shin H., Sung M., Kim J.Y. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020;35:e123. doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim E. Drawing on Israel's experience organizing volunteers to operationalize drive-through coronavirus testing centers. Disaster Med Public Health Prep. 2020 doi: 10.1017/dmp.2020.104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine Subcommittee on Standardized Collection of Race/Ethnicity Data for Healthcare Quality Improvement . In: Race, ethnicity, and language data: standardization for health care quality improvement. Ulmer C., McFadden B., Nerenz D.R., editors. National Academies Press (US); Washington (DC): 2009. [PubMed] [Google Scholar]
- 11.Coronavirus DC Data 2020. https://coronavirus.dc.gov/page/coronavirus-data Accessed December 2, 2020.
- 12.Schwamm L.H., Erskine A., Licurse A. A digital embrace to blunt the curve of COVID19 pandemic. NPJ Digit Med. 2020;3:64. doi: 10.1038/s41746-020-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.