Figure 7.
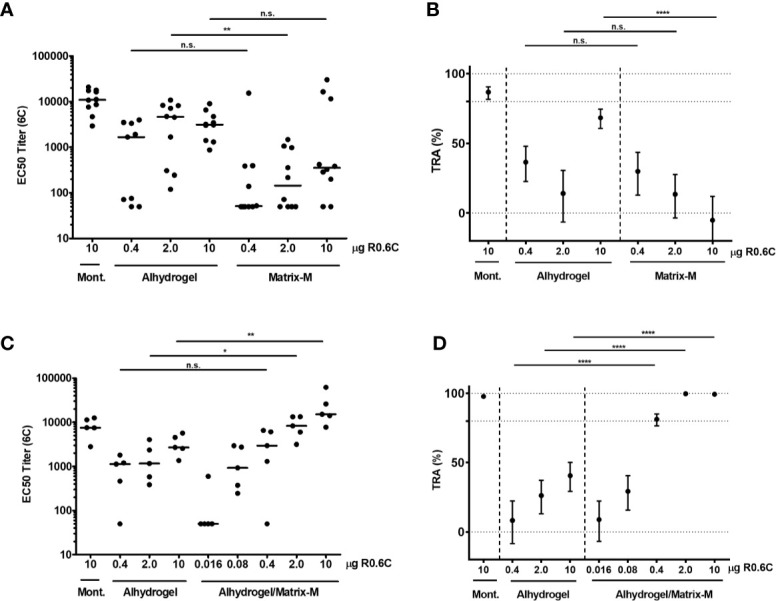
Immunogenicity of R0.6C vaccine formulations. Two separate mice immunization experiments were conducted with R0.6C formulated with different adjuvants. In each experiment one group received R0.6C formulated with Montanide (Mont.), which has previously been shown to induce high antibody titers (20). 6C-specific antibody titers for individual mice in the first (A) and second (C) experiment are shown as mid-point titers. Mid-point titers below 50 are reported as 50. Bars represent median values. Statistical difference between same-dose groups is determined by Mann-Whitney test, and reported p-values are two-sided (n.s. not significant, *p < 0.05, **p < 0.01). Pooled sera from the first immunization (B) and second immunization (D) were tested in Standard Membrane Feeding Assay (SMFA) at 1:9 dilution. Reported values and 95% confidence intervals (bars) are determined by General Linearized Mixed Models with zero-inflated negative binomial error structure and used oocyst count data from two independent SMFA experiments with 20 mosquitoes per condition and experiment. Transmission reducing activity is calculated by comparing to a non-serum control included in each SMFA. Statistical difference is determined between same-dose groups (n.s. not significant, ****p < 0.001).
