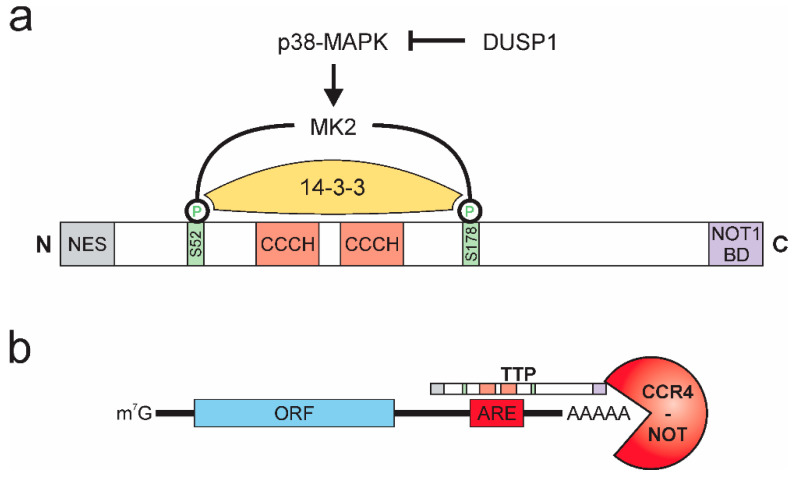
Figure 2.
Tristetraprolin (TTP) protein structure, activity regulation, and function. (a) p38-MAPK activates MK2, which phosphorylates TTP at serines 52 and 178 (mouse), which allows for binding of 14-3-3 and inactivation of TTP. DUSP 1 inhibits p38-MAPK. (NES: nuclear export signal; NOT1 BD: NOT1 binding domain) (b) TTP interacts with adenosine and uridine (AU)-rich elements (AREs) in the 3′ untranslated region of target mRNAs and recruits the CCR4-NOT deadenylation complex, facilitating rapid degradation of the target mRNA. (ORF: open reading frame).