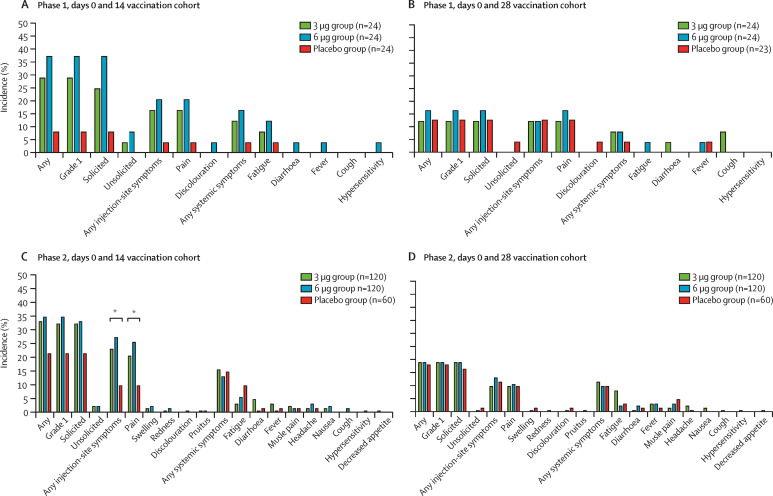
Figure 2.
Incidence of adverse reactions reported within 28 days after second dose of study drug, in the days 0 and 14 vaccination cohort in phase 1 (A) and phase 2 (C) and in the days 0 and 28 vaccination cohort in phase 1 (B) and phase 2 (D)
Adverse reactions refer to the adverse events related to the vaccination. Rare injection-site symptoms reported only in the days 0 and 14 vaccination cohort are not shown in the figure and are listed in appendix 2 along with all adverse reactions after the first and second dose (pp 4–13). *The p value of comparison among three groups is significant for the incidence of any injection-site symptoms (p=0·02) and injection-site pain (p=0·04).