Abstract
The occurrence of Austroboletus subflavidus and Fistulinella gloeocarpa is documented from the Dominican Republic. The latter species is reported for the first time outside its original locality in Martinique, extending the geographic range for this uncommon pinkish-spored bolete. A detailed morphological description is provided for each species and accompanied by color pictures of fresh basidiomes in habitat and line drawings of the main anatomical features. Both species represent independent lineages within their respective genera based on phylogenetic inference. In addition, A. subflavidus clusters in a sister lineage to the core Austroboletus clade (Austroboletus clade I) here named as Austroboletus clade II. In order to confirm the accuracy of species identification, their identity and relationships were subjected to multilocus phylogenetic analyses of three gene markers (ITS, nrLSU, RPB2) including genetic material already available in public databases. Austroboletus subflavidus is a widely distributed species in North and Central America, whereas F. gloeocarpa is apparently highly localized and seems to appear sparingly in the Dominican Republic, Martinque, and southern Florida. Comparisons with morphologically similar and molecularly inferred allied species are also presented and discussed.
Keywords: Boletales, molecular phylogeny, Greater Antilles, neotropical boletes, taxonomy
1. Introduction
With the recent advancement of molecular techniques applied to the study of boletoid mushrooms and related groups (Boletaceae, Boletales), several different generic and infrageneric lineages have been extensively investigated, revealing an extraordinary diversity mainly distributed across temperate, subtropical, and tropical environments of both hemispheres [1–8]. Yet, the increasing number of genera in the Boletaceae has barely been investigated with a molecular approach, thus determining largely unresolved phylogenetic relationships, unclear taxonomic limits, and often revealing a polyphyletic nature in their original circumscription, as in the case of the pinkish brown-spored Fistulinella Henn. and Austroboletus (Corner) Wolfe.
E.J.H. Corner first introduced Austroboletus, typified by Porphyrellus dictyotus Boedijn, as a subgenus of his broadly conceived Boletus Fr. s. l. to accommodate a number of Malaysian boletes with ornamented basidiospores [9]. A few years later, E. Horak [10] stated that “according to our personal experience with tropical species of Strobilomycetaceae at least Heimiella and subgen. Austroboletus have to be considered as good and independent genera within the taxonomic framework of the boletes". C.B. Wolfe and R.H. Petersen critically reevaluated the infrageneric limits of Porphyrellus E.-J. Gilbert s. l. and Boletus subgen. Austroboletus [11] and shortly after Wolfe [12] upgraded Austroboletus to genus rank, providing further insights into the taxonomy and a comprehensive revision of several type specimens. The recognition of Austroboletus at the generic rank was subsequently disputed by Corner [13] but accepted and integrated with additional taxa and new combinations by Pegler and Young [14], Singer [15,16], and by Horak [17,18], Watling and Gregory [19], and Singer et al. [20] based on fungal material yielded in Australasia and Latin America. Austroboletus currently comprises some 36 species [21] and incorporates taxa assigned by Singer [22] to Porphyrellus sect. Graciles Singer and sect. Tristes Singer and successively placed by Smith and Thiers [23] in Tylopilus sect. Graciles A.H. Smith and Thiers.
The genus as presently outlined is characterized from the morphological viewpoint by boletoid fruiting bodies with dry to viscid or even mucilaginous pileus and stipe surfaces, initially whitish or pale cream becoming flesh-pink to vinaceous pink or brownish pink tubular hymenophore at maturity, smooth, furfuraceous-fibrillose to more often markedly reticulate-alveolate, lacerate or lacunose stipe, generally unchanging tissues, flesh-pink, pinkish vinaceous, purplish brown, rust brown to chocolate brown spore print, variously ornamented (finely verrucose or warted to irregularly pitted but also flat-tuberculate to subreticulate) amygdaliform to ellipsoid-fusiform basidiospores, trichoderm or ixotrichoderm pileipellis, bilateral-divergent hymenophoral trama of the “Boletus-type”, gymnocarpic, velangiocarpic (primary angiocarpy), or pseudoangiocarpic (secondary angiocarpic) ontogenesis and ectomycorrhizal (ECM) association with several plant families including Fagaceae, Pinaceae, Dipterocarpaceae, Myrtaceae, and caesalpinoid legumes [8,9,12,14,16–18,20,21,24–35], although some species are suspected to be saprotrophic or only facultative ECM [32]. Austroboletus appears to be scarcely represented in temperate woodlands of both hemispheres but is particularly diverse throughout the pantropical belt, especially across the neotropical latitudes of Central and northern South America and all along the Australasian region [9,16,18,20,26,30,32–34,36].
Molecular analyses have clearly inferred a distant phylogenetic relationship of Austroboletus from Tylopilus P. Karsten s. str. and conversely an affinity with other boletoid pinkish-spored genera segregated from Tylopilus s. l., such as Fistulinella Henn., Mucilopilus Wolfe, and Veloporphyrellus L.D. Gomez & Singer and possibly with the sequestrate genus Carolinigaster M.E. Smith & S. Cruz [5,7,8,31,37–47]. The former three genera, along with Austroboletus, have been accommodated in the subfamily Austroboletoideae G. Wu & Zhu L. Yang, as they cluster in an well-delimited grouping with respect to other lineages in the Boletaceae [7]. Despite the increasing number of morphologically and molecularly-based novel species assigned to Austroboletus in the last few years from Amazon Colombia [31], India [48,49] and Australia [26], this genus has been shown to represent a polyphyletic unit [5,7,31,37,42]. Moreover, the polyphyly of Austroboletus has been further highlighted by the recent separation of the genus Ionosporus O. Khmelnitsky, based on the Malaysian species Boletus longipes Massee [41].
Fistulinella, typified by F. staudtii Henn., was first recognized by the German mycologist P. Hennings at the beginning of the twentieth century based on material recorded in Cameroon, central Africa [50]. The genus includes the species assigned by Singer [51] to Porphyrellus sect. Pseudotylopili subsect. Viscidini Singer and encompasses at present more than 20 species worldwide [21]. Fistulinella is characterized by stipitate-pileate to occasionally sequestrate fruiting bodies having relatively small size, slender and gaunt habit, velate, or evelate, usually viscid to strongly glutinous pileus and stipe surfaces, pileus sometimes scrobiculate, initially whitish becoming pinkish to vinaceous pink or brownish pink tubular hymenophore, slim stipe with a smooth, rarely reticulate but not alveolate-lacunose surface, unchanging tissues, vinaceous pink to reddish brown or rust brown to cocoa brown spore print, narrowly elongate fusoid, inamyloid to dextrinoid, smooth basidiospores, trichoderm to ixotrichoderm or ixocutis pileipellis, strongly gelatinized bilateral-divergent hymenophoral trama of the “Boletus-type”, suspected gymnocarpic ontogenesis in some species but probably also velangiocarpic (primary angiocarpy) in others and presumably but not proved ECM association with members of the Polygonaceae, Sapotaceae, Myrtaceae, Euphorbiaceae, Fagaceae, Nothofagaceae, and caesalpinoid legumes in mesophytic and hygrophytic forests ([14,16,20,21,30,32,50,52–58] this study). The biogeographic distribution of Fistulinella is more or less overlapping that of Austroboletus, the majority of species being distributed in the pantropical belt with only a few extending to temperate regions of both northern and southern hemispheres [16,32]. Despite the long-standing of Fistulinella, an unanimous taxonomic interpretation of the genus has never been reached [6,27,56]. From the phylogenetic perspective, Fistulinella is inferred to be related to Austroboletus, Mucilopilus, Veloporphyrellus, and apparently Carolinigaster [5,7,8,37–39,43,45–47] and it seems to occupy a sister position to the remainder of the Austroboletoideae [5,7,39,41,43,45,47]. On the other hand, preliminary molecular analyses suggested this genus to be polyphyletic [31,37] and accordingly an inclusive revision complemented by further sampling from different geographic regions aiming at a better understanding of its generic boundaries would be urgently needed, especially in relation to morphologically very close smooth-spored genera such as Mucilopilus and Ixechinus R. Heim ex R. Heim. Moreover Vasco-Palacios et al. [31], and Magnago et al. [42] have stressed that American species belonging in Fistulinella cluster in a statistically strongly supported separate clade with respect to those described from Australia and New Zealand, but it is not until molecular analyses are carried out on the generic type, the African taxon F. staudtii, that a taxonomic and geographic delimitation of Fistulinella s. str. lineage will be definitely clarified.
In order to reconstruct the phylogeny of Austroboletus and Fistulinella, nucleotide sequences of three regions, viz., the nuclear ribosomal internal transcribed spacer (ITS) region, large subunit nuclear ribosomal RNA gene (nrLSU) and DNA-directed RNA polymerase II subunit gene (RPB2), were generated in this study from samples of A. subflavidus and F. gloeocarpa recently recorded in the Dominican Republic (Greater Antilles). Given the limited number of mycological studies undertaken in the island, it is not at all surprising to find out distinctive bolete genera and species that were previously scarcely documented or completely overlooked.
2. Materials and methods
2.1. Collection site and sampling
Specimens examined were collected in Jarabacoa, La Vega Province and Sosúa, Puerto Plata Province, Dominican Republic, and are deposited in the Herbarium of Jardín Botánico Nacional of Santo Domingo, Dr. Rafael Ma. Moscoso, Dominican Republic (JBSD) (acronym from Thiers [59]), while “ANGE” and “MG” refer to the personal herbarium of Claudio Angelini and Matteo Gelardi, respectively. Herbarium numbers are cited for all collections from which morphological features were examined. Author citations follow the Index Fungorum, Authors of Fungal Names (www.indexfungorum.org/authorsoffungalnames.htm). Geographic distribution and morphological features of the studied species have also been checked on MyCoPortal (https://mycoportal.org) and the NYBG Boletineae project (https://sweetgum.nybg.org/science/projects/boletineae/), respectively.
2.2. Morphological studies
Macroscopic descriptions, macro-chemical reactions (30% NH4OH, 30% KOH) and ecological information, such as habitat notations, time of fruiting, and associated plant communities accompanied the detailed field notes of the fresh basidiomes. In the field, latitude, longitude, and elevation were determined with a Global Positioning System (GPS) receiver. Color terms in capital letters (e.g., White, Plate LIII) are from Ridgway [60]. Photographs of collections were taken in the natural habitat using a Nikon Coolpix 8400 camera. Microscopic anatomical features were observed and recorded from revived dried material; sections were rehydrated either in water, 5% KOH or in anionic solution saturated with Congo red. All anatomical structures were measured from preparations in anionic Congo red. Colors and pigments were described after examination in water and 5% KOH. Measurements were made at 1000× using a calibrated ocular micrometer (Nikon Eclipse E200 optical light microscope). Basidiospores were measured directly from the hymenophore of mature basidiomes, dimensions are given as (minimum) average ± standard deviation (maximum), Q = length/width ratio with the extreme values in parentheses, Qm = average quotient (length/width ratio) ± standard deviation and average spore volume was approximated as a rotation ellipsoid [V = (π.L.W2)/6 ± SD]. The notation [n/m/p] indicates that measurements were made on “n” randomly selected basidiospores from “m” basidiomes of “p” collections. The width of each basidium was measured at the widest part, and the length was measured from the apex (sterigmata excluded) to the basal septum. Radial and/or vertical sections of the pileipellis were taken midway between the center and margin of the pileus. Sections of the stipitipellis were taken from the middle part along the longitudinal axis of the stipe. Metachromatic, cyanophilic, and iodine reactions were tested by staining the basidiospores in Brilliant Cresyl blue, Cotton blue, and Melzer’s reagent, respectively. Line drawings of microstructures were traced in free hand based on digital photomicrographs of rehydrated material.
2.3. DNA extraction, PCR amplification, and DNA sequencing
DNA extraction and PCR amplification were performed from dried basidiomata (Table 1) as described by Vizzini et al. [75]. Primers ITS1F and ITS4 [76,77] were used for the ITS region; primers LR0R and LR5 [78,79] were used for the nrLSU. Amplifications of the RPB2 gene were attempted using the primers bRPB2-6F2, bRPB2-7.1R2, and bRPB2-7R2 [80,81]. The PCR products were purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI) following manufacturer’s instructions and positive reactions sequenced forward and reverse by MACROGEN Inc. (Seoul, Republic of Korea).
Table 1.
Details of specimens used in the phylogenetic analyses.
| Original name from GenBank | RPB2 | nrLSU | ITS | Specimen/voucher | Origin | Reference(s) |
|---|---|---|---|---|---|---|
| Austroboletus aff. fusisporus | KF112766 | KF112484 | – | HKAS52683 | China | Wu et al. [7] |
| Austroboletus aff. fusisporus | KF112767 | KF112486 | – | HKAS53461 | China | Wu et al. [7] |
| Austroboletus aff. mutabilis | KF112768 | KF112487 | – | HKAS53450 | China | Wu et al. [7] |
| Austroboletus aff. rostrupii | – | KJ786636 | – | G4357 | Guyana | Roy et al. [61] |
| Austroboletus amazonicus | – | KF714508 | – | 1839 AMV | Colombia | Vasco-Palacios et al. [31] |
| Austroboletus amazonicus | – | KF714509 | – | 1914 AMV | Colombia | Vasco-Palacios et al. [31] |
| Austroboletus amazonicus | – | NG_058569 | NR_153523 | HUA2032 AMV | Colombia | Vasco-Palacios et al. [31] |
| Austroboletus appendiculatus | – | – | KX530028 | KCS 1401-CAL_1304 | India | Tibpromma et al. [49] |
| Austroboletus austrovirens | – | – | KP242207 | BRI:AQ0794143 | Australia | Fechner et al. [26] |
| Austroboletus austrovirens | KP242133 | KP242227 | KP242208 | BRI:AQ0794171 | Australia | Bonito et al. (unpubl.) |
| Austroboletus austrovirens | KP242131 | KP242226 | KP242209 | BRI:AQ0794609 | Australia | Bonito et al. (unpubl.) |
| Austroboletus austrovirens | – | – | KP242210 | BRI:AQ0794622 | Australia | Fechner et al. [26] |
| Austroboletus austrovirens | KP242130 | KP242225 | KP242211 | BRI:AQ0795791 | Australia | Fechner et al. [26] |
| Austroboletus austrovirens | – | – | KP242212 | BRI:AQ0796003 | Australia | Fechner et al. [26] |
| Austroboletus austrovirens | KP242113 | KP242284 | KP012789 | MEL:2382920a | Australia | Bonito et al. (unpubl.) |
| Austroboletus austrovirens | – | – | KP242214 | MEL:2382920b | Australia | Fechner et al. [26] |
| Austroboletus cf. gracilis | – | MN174791 | MN174796 | JLF6600 | USA | Frank (unpubl.) |
| Austroboletus cf. novae-zelandiae | – | KC552061 | – | CD567 | Australia | Orihara et al. [62] |
| Austroboletus cf. subvirens | MH614752 | – | – | OR0573 | Thailand | Vadthanarat et al. [63] |
| Austroboletus dictyotus | – | JX901138 | – | HKAS59804 | China | Hosen et al. [64] |
| Austroboletus festivus | – | – | KT724085 | AMV1800 | Colombia | Vasco-Palacios et al. (unpubl.) |
| Austroboletus festivus | – | KT724095 | KT724086 | AMV1881 | Colombia | Vasco-Palacios et al. (unpubl.) |
| Austroboletus festivus | – | KY888001 | KY886202 | FLOR:51599 | Brazil | Magnago et al. [42] |
| Austroboletus fusisporus | – | AB509830 | 122–549 | Japan | Sato et al. (unpubl.) | |
| Austroboletus fusisporus | – | JX889720 | JX889719 | HKAS75207 | China | Hosen et al. [64] |
| Austroboletus fusisporus | – | MK765810 | JXSB0351 | China ? | Chen (unpubl.) | |
| Austroboletus gracilis | – | – | MH465078 | ACAD11344F | Canada | Young et al. [65] |
| Austroboletus gracilis | – | – | MH167935 | Mushroom Observer # 310751 | Mexico | Rockefeller (2018, direct submission) |
| Austroboletus gracilis | – | – | MH979242 | NAMA 2017-106 | USA | Russell (2018, direct submission) |
| Austroboletus gracilis | – | EU522815 | – | TM03_434 | Canada | Porter et al. [66] |
| Austroboletus gracilis var. flavipes | – | MK601714 | – | CFMR BOS-562 | USA | Kuo and Ortiz-Santana [5] |
| Austroboletus gracilis var. gracilis | MK766277 | MK601715 | CFMR BOS-547 | USA | Kuo and Ortiz-Santana [5] | |
| Austroboletus lacunosus | KP242090 | KP242272 | KP242161 | BRI:AQ0795787 | Australia | Bonito et al. (unpubl.) |
| Austroboletus lacunosus | – | KC552056 | KC552014 | MEL:2233764 | Australia | Orihara et al. [62] |
| Austroboletus lacunosus | – | KC552057 | KC552015 | MEL:2265009 | Australia | Orihara et al. [62] |
| Austroboletus lacunosus | – | – | KP191804 | PDD:83019 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus lacunosus | – | JX889669 | – | REH9146 | Australia | Halling et al. [67] |
| Austroboletus mucosus | – | AY612798 | – | TH6300 | – | Drehmel et al. [3] |
| Austroboletus mutabilis | KP242097 | KP242266 | KP242167 | BRI:AQ0669270 | Australia | Bonito et al. (unpubl.) |
| Austroboletus mutabilis | KP242098 | KP242263 | KP242169 | BRI:AQ0795793 | Australia | Bonito et al. (unpubl.) |
| Austroboletus mutabilis | KP242099 | KP242262 | KP242170 | BRI:AQ0796266 | Australia | Bonito et al. (unpubl.) |
| Austroboletus neotropicalis | – | JQ924334 | JQ924301 | NY181457 | Costa Rica | Wu et al. (unpubl.) |
| Austroboletus niveus | – | KC552058 | KC552016 | MEL:2053830 | Australia | Orihara et al. [62] |
| Austroboletus niveus | KP242109 | KP242279 | KP242217 | Perth 6660703 | Australia | Bonito et al. (unpubl.) |
| Austroboletus niveus | – | JX889668 | – | REH9487 | Australia | Halling et al. [67] |
| Austroboletus niveus | – | KP191672 | KP191800 | PDD:105213 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus niveus | – | KP191673 | KP191801 | PDD:105246 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus niveus | – | – | KP191802 | PDD:81219 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus niveus | – | DQ534622 | – | Strain 312 | New Zealand | Binder and Hibbett [2] |
| Austroboletus novae-zelandiae | – | KP242256 | KP242175 | MEL:2370154 | Tasmania (Australia) | Bonito et al. (unpubl,) |
| Austroboletus novae-zelandiae | – | KP191671 | KP191803 | PDD:105097 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus novae-zelandiae | – | HM060327 | PDD:72542 | New Zealand | Johnston and Park (unpubl.) | |
| Austroboletus novae-zelandiae | – | DQ534623 | – | Strain 50 | New Zealand | Binder and Hibbett [2] |
| Austroboletus occidentalis | – | KC552059 | KC552017 | MEL:2300518 | Australia | Orihara et al. [62] |
| Austroboletus rarus | KP242086 | KP242236 | KP242197 | BRI:AQ0794045 | Australia | Bonito et al. (unpubl.) |
| Austroboletus rionegrensis | – | – | KY886201 | INPA 78693 | Brazil | Magnago et al. [42] |
| Austroboletus roseialbus | – | KY872650 | KY872653 | Dodd | Australia | Fechner et al. [26] |
| Austroboletus roseialbus | – | KY872651 | KY872652 | REH10024 | Australia | Fechner et al. [26] |
| Austroboletus rostrupii | KP242089 | – | KP242160 | BRI:AQ0795785 | Australia | Bonito et al. (unpubl.) |
| Austroboletus rostrupii | – | – | JN168683 | TH8189 | Guyana | Smith et al. [68] |
| Austroboletus sp. | KP242115 | KP242235 | – | BRI:AQ0794156 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | KP242106 | KP242234 | KP242215 | BRI:AQ0794222 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | KP242087 | – | KP242158 | BRI:AQ0794242 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | KP242102 | KP242259 | – | BRI:AQ0794271 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | KP242094 | – | KP242159 | BRI:AQ0794272 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | – | KP242283 | KP242213 | MEL:2382826 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | – | – | KY774008 | CY13_008 | New Caledonia | Carriconde et al. (unpubl.) |
| Austroboletus sp. | – | – | KY774007 | CYMy36L1 | New Caledonia | Carriconde et al. (unpubl.) |
| Austroboletus sp. | – | KF030351 | – | DPL7541 | USA | Nuhn et al. [6] |
| Austroboletus sp. | KF112764 | KF112383 | – | HKAS:57756 | China | Wu et al. [7] |
| Austroboletus sp. | KF112765 | KF112485 | – | HKAS:59624 | China | Wu et al. [7] |
| Austroboletus sp. | KT990367 | KT990527 | – | HKAS74743 | China | Wu et al. [8] |
| Austroboletus sp. | – | KY090995 | – | LAM 0222 | Malaysia | Peay and Lim (unpubl.) |
| Austroboletus sp. | – | KY091070 | – | LAM 0479 | Malaysia | Peay and Lim (unpubl.) |
| Austroboletus sp. | KP242134 | KC552060 | KP242203 | MEL:2305143 | New Caledonia | Orihara et al. [62] |
| Austroboletus sp. | MH614753 | – | – | OR0891 | Thailand | Vadthanarat et al. [63] |
| Austroboletus sp. | – | KP191670 | KP191805 | OTA FUNNZ 2013434 | New Zealand | Lebel and Cooper (unpubl.) |
| Austroboletus sp. | KP242126 | KP242277 | KP242216 | Perth 06658407 | Australia | Bonito et al. (unpubl.) |
| Austroboletus sp. | – | KP242285 | – | Perth 7660928 | Australia | Bonito et al. (unpubl.) |
| Austroboletus subflavidus | MT590754 | MT580902 | MT581525 | JBSD130771 (ANGE108 and MG775) | Dominican Republic | This study |
| Austroboletus subflavidus | MT590755 | MT580903 | MT581526 | JBSD130772 (ANGE388 and MG776) | Dominican Republic | This study |
| Austroboletus subflavidus | – | MT580901 | MT581523 | CFMR:DR2859; isolate = TJB-9787 | Dominican Republic | This study |
| Austroboletus subflavidus | – | – | MT581524 | CFMR:DR592; isolate = DJL-DR-48 | Dominican Republic | This study |
| Austroboletus subflavidus | – | – | MT581522 | CFMR:BZ1824; isolate = DJL-BZ-27 | Belize | This study |
| Austroboletus subflavidus | MK766278 | MK601716 | – | CFMR BZ-3178 BOS-625 | Belize | Kuo and Ortiz-Santana [5] |
| Austroboletus subflavidus | – | MT580900 | MT581521 | CFMR:BOTH-3463 | Florida (USA) | This study |
| Austroboletus subflavidus | – | – | MH016816 | FLAS-F-60635 | Florida (USA) | Kaminsky et al. (unpubl.) |
| Austroboletus subvirens | – | – | AB509915 | 120-707 | Japan | Sato et al. (unpubl.) |
| Austroboletus subvirens | – | JN378518 | – | KPM-NC-0017836 | Japan | Orihara et al. [69] |
| Austroboletus viscidoviridis | KP242128 | KP242282 | KP242219 | Perth 7588682 | Australia | Bonito et al. (unpubl.) |
| Austroboletus viscidoviridis | – | – | KY872649 | REH9993 | Australia | Fechner et al. [26] |
| Bothia castanella | – | DQ867117 | DQ867110 | MB03-053 | USA | Halling et al. [70] |
| Bothia fujianensis | – | KM269193 | KM269195 | HKAS82694 | China | Zeng et al. [71] |
| Fistulinella campinaranae | – | KY888003 | KY886204 | FLOR:51608 | Brazil | Magnago et al. [42] |
| Fistulinella campinaranae var. scrobiculata | – | KT724100 | KT724090 | AMV1513 | Colombia | Vasco-Palacios et al. [31] |
| Fistulinella cinereoalba | – | GQ477439 | KT339237 | TH8471 | Guyana | Fulgenzi et al. [27] |
| Fistulinella gloeocarpa | MT59076 | MT580906 | MT581527 | JBSD130769 (ANGE969 and MG777) | Dominican Republic | This study |
| Fistulinella gloeocarpa | – | MT580904 | – | CFMR:B4 | The Bahamas | This study |
| Fistulinella gloeocarpa | – | MT580905 | – | CFMR:B10 | The Bahamas | This study |
| Fistulinella gloeocarpa | – | – | GQ981503 | KM162946 | The Bahamas | Bidartondo and Doring (unpubl.) |
| Fistulinella olivaceoalba | – | MH745969 | – | HKAS53432 | Vietnam | Crous et al. [37] |
| Fistulinella olivaceoalba | – | MH718396 | NR_163311 | LE312004 | Vietnam | Crous et al. [37] |
| Fistulinella prunicolor | MG212630 | JX889648 | – | REH9502 | Australia | Halling et al. [67] |
| Fistulinella ruschii | – | KY888004 | KY886205 | FLOR:51609 | Brazil | Magnago et al. [42] |
| Fistulinella ruschii | – | NG_060432 | NR_156320 | FLOR:51611 | Brazil | Magnago et al. [42] |
| Fistulinella ruschii | – | KY888005 | KY886209 | ICN 192818 | Brazil | Magnago et al. [42] |
| Fistulinella ruschii | – | MT580907 | – | CORT:TJB-8329 | United States Virgin Islands | This study |
| Fistulinella sp. | – | – | KF878352 | AMV511 | Colombia | Vasco-Palacios et al. [31] |
| Fistulinella viscida | – | HM624054 | – | PDD 25185 | New Zealand | Li and Yang (unpubl.) |
| Fistulinella viscida | – | AF456826 | – | Strain 238 | – | Binder and Bresinsky [72] |
| Mucilopilus castaneiceps | KT990391 | KT990555 | – | HKAS50338 | China | Wu et al. [8] |
| Mucilopilus castaneiceps | KT990385 | KT990547 | – | HKAS71039 | China | Wu et al. [8] |
| Mucilopilus castaneiceps | KF112735 | KF112382 | – | HKAS75045 | China | Wu et al. [7] |
| Solioccasus polychromus | – | JQ287643 | JX888459 | J. Trappe 15399 | Australia | Trappe et al. [73] |
| uncultured Fistulinella | – | – | KT757689 | uncultured clone AMV511root | Colombia | Vasco-Palacios et al. (unpubl.) |
| Veloporphyrellus aff. velatus | KF112733 | KF112380 | – | HKAS57490 | China | Wu et al. [7] |
| Veloporphyrellus alpinus | – | JX984537 | – | KUN:HKAS57490 | China | Li et al. [74] |
| Veloporphyrellus conicus | – | JX984543 | – | CFMR:BZ1670 | Belize | Li et al. [74] |
| Veloporphyrellus conicus | MH614792 | – | – | REH8510 | Belize | Vadthanarat et al. [63] |
| Veloporphyrellus pantoleucus | – | JX984547 | – | F:Gomez21232 basidiocarp1 | Costa Rica | Li et al. [74] |
| Veloporphyrellus pseudovelatus | – | JX984540 | – | KUN:HKAS52258 | China | Li et al. [74] |
| Veloporphyrellus velatus | – | JX984546 | – | KUN:HKAS63668 | China | Li et al. [74] |
| Veloporphyrellus vulpinus | – | MN511171 | MN511178 | LE315547 | Viet Nam | Crous et al. [37] |
Newly obtained sequences are in bold.
2.4. Sequence alignment, data set assembly, and phylogenetic analyses
The sequences obtained in this study were checked and assembled using Geneious v. 11.1.4 [82] and compared to those available in GenBank by using the Blastn algorithm [83]. Chromatograms were examined and manually edited for accuracy. Newly acquired sequences were submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and samples with accession numbers are listed in bold typeface in Table 1. Homologous sequences from vouchered specimens and from environmental samples were selected and retrieved from GenBank (see Table 1).
Alignments were generated for the ITS, nrLSU, and RPB2 datasets with MAFFT [84] with default conditions for gap openings and gap extension penalties. Alignments were then manually adjusted and concatenated using Geneious v. 11.1.4 [82]. We estimated the best fit substitution model for each single alignment using the Bayesian information criterion (BIC) with jModelTest 2 [85] and therefore selected the TIM1 + G, TIM2 + G, and K80 + G models for nrLSU, ITS, and RPB2, respectively. The ITS dataset was not partitioned. A combined nrLSU/ITS/RPB2 analyses focused on the Austroboletoideae as circumscribed by Wu et al. [7,8] was performed. Sequences of Austroboletus betulae [3,86] were not included in the analyses because the species was recently inferred to belong in Aureoboletus Pouzar within the subfamily Xerocomoideae [5]. Bothia and Solioccasus sequences were used as outgroup according to Wu et al. [7,8] and Magnago et al. [42]. Phylogenetic trees were constructed with Bayesian inference (BI) and Maximum likelihood (ML) criteria. The partitioned BI was performed with MrBayes v. 3.2.7a [87] with one cold and three incrementally heated simultaneous Monte Carlo Markov chains (MCMC) run for 10 M generations, under the selected evolutionary models for each unlinked partition. Two simultaneous runs were performed independently. Trees were sampled every 1000 generations, resulting in sampling of 10001 trees per single run with the first 2500 trees (25%) discarded as burn-in. For the remaining trees of the two independent runs, a majority rule consensus tree showing all compatible partitions was computed to obtain estimates for Bayesian posterior probabilities (BPPs). Partitioned ML analyses were performed using RAxML v. 7.3.2 [88] with 1000 bootstrap replicates [89] and the GTRGAMMA model of sequence evolution. Support values from bootstrapping runs (MLB) were mapped on the best ML tree using the “-f a” option of RAxML and “-x 12345” as a random seed to invoke the novel rapid bootstrapping algorithm. BI and ML analyses were run on the CIPRES Science Gateway [90]. BPP values ≥0.95 and MLB values ≥70%, are reported in the resulting tree (Figure 1). Lower values are exceptionally represented inside parentheses. Branch lengths were estimated as mean values over the sampled trees. Pairwise percent identity values (P %IV) of the ITS sequences were calculated using Geneious v. 11.1.4 [82]. Alignments and phylogenetic trees are available at TreeBASE (www.treebase.org) under ID 26454.
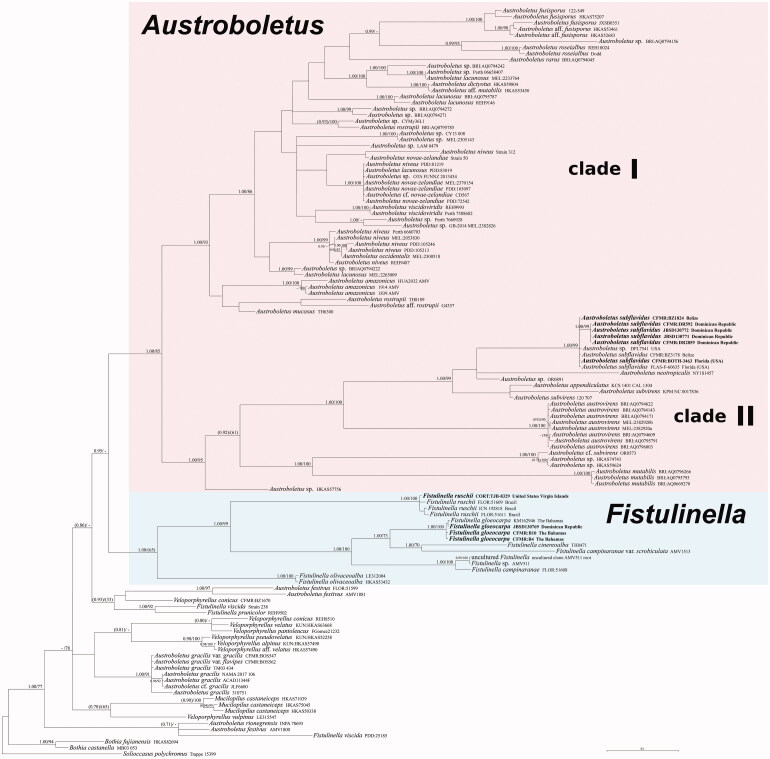
Figure 1.
Phylogeny of the genera in Austroboletoideae based on a Bayesian and Maximum likelihood inference analyses of a combined matrix of three nuclear gene regions (nrLSU, ITS, and RPB2). Bayesian posterior probability (BPP) values (in bold) ≥0.95 and Maximum likelihood bootstrap (MLB) values ≥70% are shown on the branches. Lower values are exceptionally represented inside parentheses. Newly sequenced collections are in bold.
3. Results
3.1. Molecular analyses
Both Bayesian and Maximum Likelihood analyses produced comparable topologies and therefore only Bayesian trees with BPP and MLB values are shown (Figure 1). The nrLSU dataset comprised 92 accessions and 974 characters. The ITS dataset included 76 taxa and 1397 characters. The RPB2 dataset is composed of 39 taxa and 527 characters. The combined dataset comprised 122 specimens (Table 1). The genera Austroboletus and Fistulinella, as currently morphologically circumscribed, are polyphyletic, as well as Veloporphyrellus (Figure 1). Two major strongly supported sister clades were recognized in Austroboletus, herein named as I (BPP = 1; MLB = 93%), including the type species A. dictyotus, and II (BPP = 1; MLB = 95%). Austroboletus festivus, A. gracilis, and A. rionegrensis are independent evolutionary lineages outside Austroboletus. The sequences of A. subflavidus form a separate clade (BPP = 1; MLB = 99%) within major clade II. P%IV of the ITS sequences of the A. subflavidus clade is 97.3.
Most Fistulinella sequences cluster in a clade strongly supported only by the Bayesian analyses (BPP = 1; MLB = 65%) also including F. gloeocarpa. Fistulinella prunicolor and F. viscida fall outside the Fistulinella clade. The two Fistulinella gloeocarpa collections show a P%IV of 99.4.
3.2. Taxonomy
Austroboletus subflavidus (Murrill) Wolfe, Bibliotheca Mycologica 69: 67. 1979 (“1980”) Figures 2 and 3.
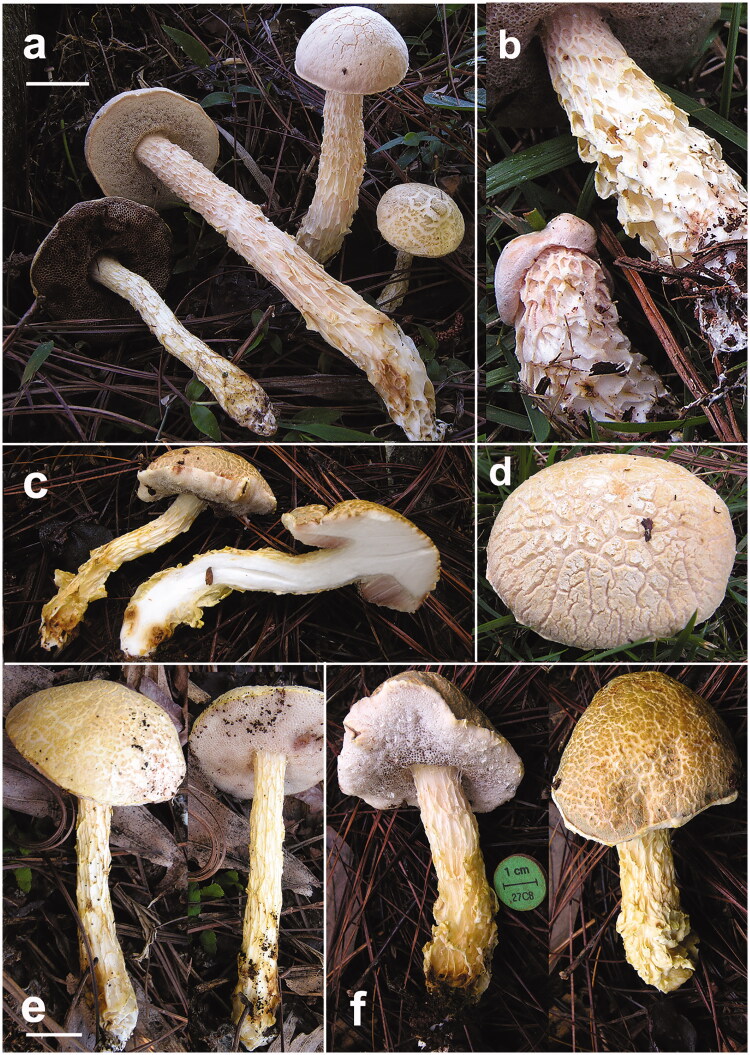
Figure 2.
Austroboletus subflavidus basidiomes in habitat. (a) JBSD130773 (ANGE1145); (b–d) close up of the stipe, context, and pileus, respectively (b, d: JBSD130774, ANGE1146; c: JBSD130771, ANGE108); (e) JBSD130772 (ANGE388); (f) JBSD130771 (ANGE108). Scale bars: 1 cm. Photos by C. Angelini.
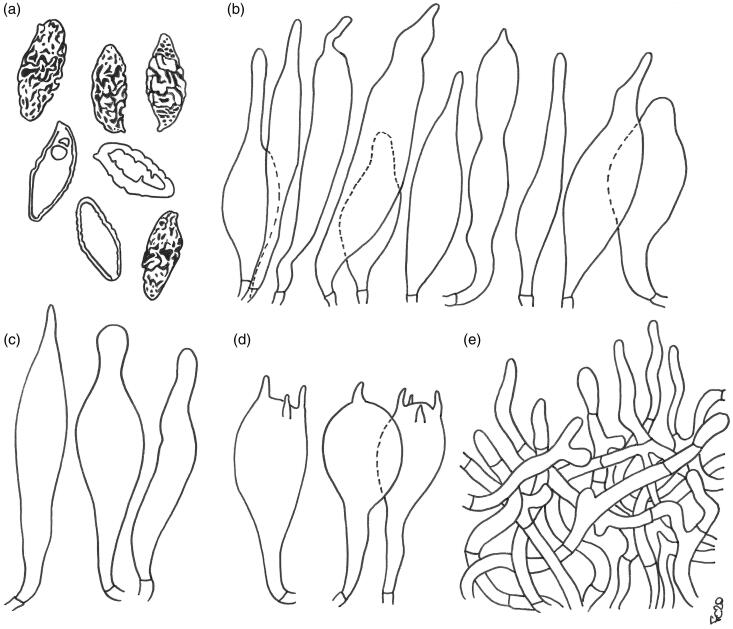
Figure 3.
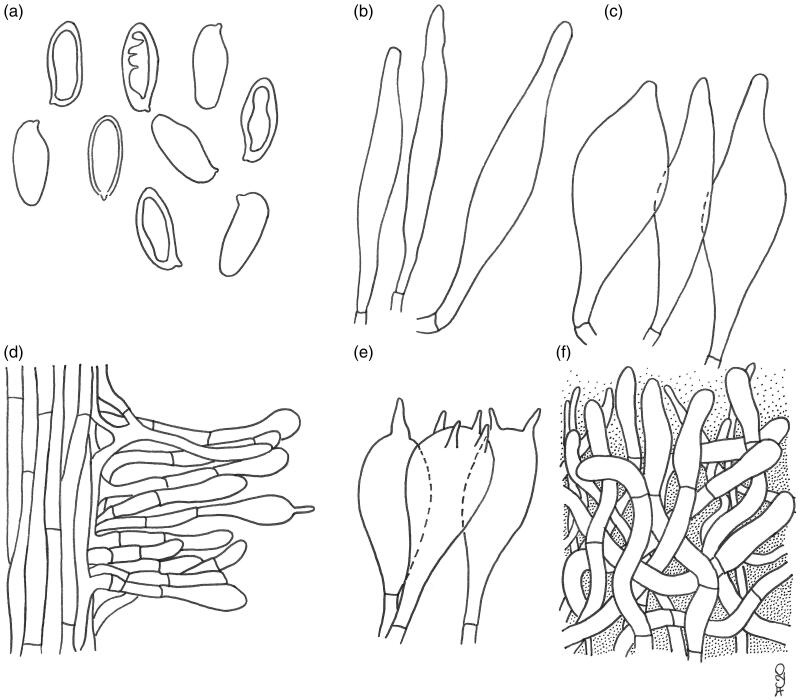
Austroboletus subflavidus. Micromorphological features; (a) basidiospores; (b) cheilo- and pleurocystidia; (c) caulocystidia; (d) basidia; (e) elements of the pileipellis. Scale bars: 10 μm (a–d); 20 μm (e). Drawings by F. Costanzo.
MYCOBANK MB 118437
Basionym: Tylopilus subflavidus Murrill, Mycologia 30 (5): 521. 1938.
≡Boletus subflavidus (Murrill) Murrill, Mycologia 30 (5): 525. 1938
≡Boletellus subflavidus (Murrill) Snell, Mycologia 33 (4): 422. 1941.
≡Porphyrellus subflavidus (Murrill) Singer, Farlowia 2 (1): 120. 1945.
Holotype: USA, Florida, Gainesville, under Pinus sp., 14 Aug 1937, W.A. Murrill, 15862 (FLAS); neotype designated by C.B. Wolfe [12]: USA, Florida, Gainesville, 11 Jul 1938, E. West, Arnold and W.A. Murrill (NY, isoneotype: FH); authentic material also preserved in NY and FLAS [91].
Basidiomes small. Pileus (1.4) 2.1–5.0 (5.5) cm broad, at first hemispherical then persistenly convex to nearly applanate, not depressed at center, regularly to hardly unevenly shaped by shallow depressions, moderately fleshy, firm at the beginning but progressively softer with age; margin obtuse, steady to faintly wavy-lobed, slightly involute then curved downwards, sterile and not or only a little extending beyond the tubes (up to 1 mm); surface matt, dry, very finely tomentose, soon disrupted and appearing tipically areolate with age and showing the whitish (White, Pl. LIII) context beneath, rarely not cracked; cuticle patches color ranging from whitish, ivory, beige or pale cream yellowish (White, Pl. LIII; Maize Yellow, Martius Yellow, Pl. IV; Marguerite Yellow, Pl. XXX; Naphtalene Yellow, Straw Yellow, Pl. XVI) to ochraceous or pale ochraceous-olive (Deep Olive-Buff, Dark Olive-Buff, Pl. XL; Primuline Yellow, Olive Lake, Buffy Citrine, Pl. XVI; Ecru Olive, Light Yellowish Olive, Isabella Color, Buffy Olive, Pl. XXX); not staining on handling or when injured; subcuticular layer white (White, Pl. LIII). Tubes at first thin then increasingly broader, initially shorter or as long as but later longer than the thickness of pileus context (up to 1.4 cm long), adnate at first but soon deeply depressed around the stipe apex, whitish (White, Pl. LIII) at first to pale flesh-pink (Flesh-Pink, Venetian Pink, Pl. XIII; Pale Salmon Color, Pl. XIV; Pale Purplish Vinaceous, Pale Grayish Vinaceous, Pl. XXXIX), then pinkish lilac (Pale Lavander Violet, Pale Mauve, Mauvette, Light Mauve, Pl. XXV; Light Pinkish Lilac, Pl. XXXVII; Pale Brownish Vinaceous, Pl. XXXIX) and finally brownish pink to dirty brownish (Sorghum Brown, Hay’s Brown, Light Seal Brown, Pl. XXXIX), unchangeable when cut. Pores initially forming a flat surface, later convex to ascendant, at first small then gradually wider (up to 1 mm in diam.), simple, roundish to barely angular at maturity, concolorous with or slightly paler than tubes and very slowly and faintly darkening (Purplish Vinaceous, Livid Brown, Pl. XXXIX) on bruising or when injured, occasionally beaded by scattered watery droplets. Stipe (2.9) 4.5–7.5 (10.2) × (0.4) 0.6–1.8 (2.0) cm, constantly longer than pileus diameter, central to slightly off-center, solid, firm, dry but decidedly viscid with moist weather, straight or curved, cylindrical to more frequently gradually swollen toward the base, ending with a short taproot at the very base, apparently evelate; surface prominently reticulate to deeply reticulate-alveolate throughout, reticulate pattern consisting of longitudinally stretched, waxy anastomosing ribs, increasingly coarser and more prominent to distinctly folded toward the base; whitish (White, Pl. LIII) to ivory or beige (Maize Yellow, Pl. IV; Marguerite Yellow, Pl. XXX) in the upper three fourth, pale cream yellowish to ochraceous (Martius Yellow, Pl. IV; Naphtalene Yellow, Straw Yellow, Pl. XVI; Primuline Yellow, Pl. XVI) downwards, usually with pale brown (Chamois, Pl. XXX; Sudan Brown, Pl. III) spots or shades at the stipe base, reticulum concolorous to pale cream yellowish (Martius Yellow, Pl. IV; Naphtalene Yellow, Straw Yellow, Pl. XVI), unchangeable when pressed; basal mycelium white (White, Pl. LIII). Context firm when young, later soft textured and eventually flabby in the pileus (up to 2.2 cm thick in the central zone), a little more fibrous in the stipe, white (White, Pl. LIII) throughout, usually with pale brown (Chamois, Pl. XXX; Sudan Brown, Pl. III) spots or shades at the stipe base; unchangeable when exposed to air; subhymenophoral layer white (White, Pl. LIII); exsiccate pileus beige to pale olive brown (Maize Yellow, Pl. IV; Marguerite Yellow, Pl. XXX; Dark Olive-Buff, Pl. XL), hymenophore dull brown (Dull Brown, Pl. XXX), stipe and context beige (Maize Yellow, Pl. IV; Marguerite Yellow, Pl. XXX). Odor indistinct to faintly fruity. Taste bitter. Spore print not obtained. Macrochemical spot-test reactions: 30% KOH: none; 25% NH4OH: pinkish on pileus, none on context.
Basidiospores [122/7/4] (13.1) 15.9 ± 1.15 (19.5) × (5.5) 7.0 ± 0.58 (8.7) µm, Q = (1.76) 1.87–2.61 (2.68), Qm = 2.26 ± 0.16, V = 416 ± 89 µm³ (including ornamentation), inequilateral, ellipsoid-fusiform, ellipsoid to broadly ellipsoid in side view, broadly ellipsoid to amygdaliform in face view, distinctly verrucose in central part by disruption of the outer wall, minutely pitted or furrowed to form irregular isolated, short rounded-tuberculate warts, or sinuous confluent meandering ridges 0.1–0.7 µm high, becoming progressively less pronounced toward both the apex and the distal end which appear minutely perforate-punctate or porose to nearly smooth, apex rounded, with a short apiculus and usually with a less ornamented suprahilar applanation or shallow depression, often with a shallow abaxial depression close to the distal end and with an adaxial swelling, moderately thick-walled (0.5–1.0 µm), honey yellow colored in water, and 5% KOH, having one, less frequently two or three large oil droplets when mature, rarely pluri-guttulate, inamyloid to faintly dextrinoid, strongly cyanophilic, and with a weak metachromatic reaction. Basidia 27–49 (51) × 12–19 µm (n = 18), subclavate to clavate or broadly clavate, moderately thick-walled (0.3–0.8 µm), predominantly 4-spored but also 1-, 2-, or 3-spored, usually bearing relatively short sterigmata (2–5 µm) (sterigmata up to 6 µm long in 1-spored basidia), hyaline to pale yellowish and seldom containing scattered straw-yellow oil guttles in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without basal clamps; basidioles faintly clavate to clavate, similar in size to basidia. Cheilocystidia (33) 35–65 (70) × 7–10 (12) µm (n = 12), uncommon, moderately slender, projecting straight to sometimes flexuous, irregularly cylindrical or cylindrical fusiform to fusiform with a narrow and long neck, sometimes mucronate, less frequently ventricose fusiform, with rounded to subacute tip, smooth, moderately thick-walled (0.5–0.8 µm), hyaline to pale yellowish in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without epiparietal encrustations. Pleurocystidia (36) 43–69 (73) × 8–12 µm (n = 9), infrequent, size, shape, color, and chemical reactions similar to cheilocystidia, occasionally lageniform, subclavate, mucronate to subcapitate. Pseudocystidia not recorded. Pileipellis a trichoderm consisting of strongly interwoven, elongated, frequently branched, filamentous and sinuous to cylindrical hyphae not to moderately embedded in gelatinous matter; terminal elements 27–100 × 4–15 µm, long and slender, filamentous and sinuous or short cylindrical to cystidioid, apex rounded-obtuse to more rarely pointed, thick-walled (up to 1.3 µm), hyaline to pale straw yellow in water and 5% KOH, golden yellow (inamyloid) in Melzer’s, smooth to occasionally ornamented by a very subtle granular epiparietal encrustation; subterminal elements similar in shape, size, and color to terminal elements. Stipitipellis a layer of slender, parallel to loosely intermingled and longitudinally running, smooth-walled, adpressed hyphae, 2–11 µm wide, hyaline to very pale yellowish in water and 5% KOH; the stipe apex covered by a layer 300–400 µm thick of strongly entangled filamentous and sinuous, frequently branched hyphae 2–6 µm broad, having a wall up to 0.3 µm thick, heavily embedded in gelatinous matter, giving rise in the outermost part to a well-developed caulohymenial layer consisting of caulobasidioles, projecting caulocystidia similar in shape, size, color and chemical reactions to hymenial cystidia, (50) 53–57 × (8) 10–14 µm (n = 5), having a wall up to 0.8 µm thick and very sparse caulobasidia mostly 1-, 2-, and 3-spored, 44–51 × 10–14 µm, sterigmata up to 6 µm long (n = 3). Lateral stipe stratum under the caulohymenium usually absent or not differentiated from the underlying layer but occasionally present, of the “boletoid type”, 30–40 µm thick and consisting of divergent, inclined and running toward the external surface, loosely intermingled and branched hyphae remaining separate and heavily embedded in a gelatinous substance. Stipe trama composed of densely arranged, subparallel to moderately interwoven, filamentous, smooth, inamyloid hyphae, 3–16 µm broad. Hymenophoral trama bilateral divergent of the “Boletus-type”, with slightly to strongly divergent, recurved-arcuate and loosely arranged, not-branched, distantly septate and generally not restricted at septa, gelatinous hyphae (lateral strata hyphae in transversal section not touching each other, (3) 4–8 (9) µm apart, 3–10 µm broad), hyaline to very pale yellowish in water and 5% KOH, inamyloid in Melzer’s; lateral strata (20) 30–40 (50) µm thick, mediostratum (15) 20–30 (40) µm thick, axially arranged, consisting of a tightly adpressed, non-gelatinous bundle of hyphae, 3–8 µm broad, more frequently septate; in Congo Red the mediostratum is darker than the lateral strata. Oleipherous hyphae scattered although more frequently observed in the hymenium and basal stipe trama, golden yellow in 5% KOH and Melzer’s. Clamp connections absent in all tissues. Ontogenetic development probably gymnocarpic.
Edibility unknown.
Ecology and phenology: solitary to scattered or gregarious, growing on soil among litter in association with Pinus occidentalis in the Dominican Republic. Elsewhere associated with other pine trees (P. palustris, P. caribaea, etc.) and oaks (Quercus marilandica, Q. minima, Q. laurifolia, Q. virginiana, Q. oleoides, Q. humboldtii, etc.). Apparently uncommon at least in the Dominican Republic, fairly common to infrequent or occasional elsewhere. June to January.
Known distribution: eastern North America, eastern, and south-eastern USA (New Jersey south to Florida and west to Texas) down into the Gulf coastal plain and Mexico, Belize and Costa Rica in mainland Central America south to Colombia in northern South America, in the Greater Antilles Islands of the Caribbean reported from the Dominican Republic.
Examined material: DOMINICAN REPUBLIC, La Vega Province, Jarabacoa, Buena Vista, 19°11′09.3″N 70°35′16.9″W, 660 m, 22 Dec 2013, a single mature specimen, under P. occidentalis, C. Angelini (JBSD130771, ANGE108, and MG775); same loc., 06 Dec 2014, a single middle-aged specimen, C. Angelini (JBSD130772, ANGE388, and MG776); same loc., Golf Club, 19°11′12.5″N 70°35′25.5″W, 800 m, 03 Jan 2020, several specimens in all developmental stages, C. Angelini (JBSD130773 and ANGE1145); same loc., 03 Jan 2020, three specimens two of which mature and the other one a primordium, C. Angelini (JBSD130774 and ANGE1146).
Comments: Originally described from northern Florida as a member of Tylopilus by Murrill [92], the species was then recombined in Boletellus Murrill by Snell [93] and subsequently transferred to Porphyrellus E.-J. Gilbert by Singer [94]. Some decades later Wolfe [12] placed it in Austroboletus where it is currently retained based on morphological and molecular inference.
Austroboletus subflavidus is readily distinguished among congeneric species based on the small to medium-sized basidiomes (pileus up to 11 cm diam. and stipe up to 14.5 cm long and 5 cm wide), pileus surface dry and becoming rimose-areolate with age, whitish beige or pale cream yellowish to ochraceous olive, occasionally with a pale pinkish tinge, pinkish hymenophore, slender, deeply reticulate-alveolate, whitish beige to yellowish stipe usually showing brownish shades or patches at the base, white context and basal mycelium, unchanging tissues on bruising or injury, bitterish to bitter taste, ellipsoid-fusiform to amygdaliform, strongly cyanophilic basidiospores which are minutely pitted forming short round-warted or meandering fissured-ridged medial ornamentation and becoming rugulose-punctate to nearly smooth proximally and distally, trichoderm pileipellis consisting of filamentous to cylindrical hyphae and the occurrence in temperate to tropical environments in association with Fagaceae and Pinaceae [12,14,24,25,29,51,92,94–99]. In mainland regions A. subflavidus is usually found under a wide array of pine (belonging to both Pinus subgen. Pinus and P. subgen. Strobus) and oak trees [20,29,94,96,98,100]. In the Dominican Republic, it appears to be associated exclusively with five-needled P. occidentalis in mountain woodlands ([29]; this study).
When compared with congeneric American species, A. subflavidus is practically unmistakable but reveals a slight resemblance with other extralimital pale colored Austroboletus, such as A. niveus (G. Stev.) Wolfe, A. eburneus Watling & N.M. Greg., A. roseialbus Fechner, Bonito, Lebel, & Halling and A. appendiculatus Semwal et al.
Confident morphological identification criteria for distinguishing A. niveus from A. subflavidus include viscid pileus and stipe surface with age, slightly longer and narrower, elongate subfusiform to cylindrical basidiospores [(14.5) 17–19 (21.8) × (4) 4.5–6.0 (6.8) µm] with a very subtle granular punctate, rugulose ornamentation distributed over the entire surface, lageniform, broader hymenial cystidia (52–75 × 12–23 µm), no staining reaction with NH4OH on pileus and the occurrence with Agathis (Araucariaceae), Nothofagus (Nothofagaceae), Eucalyptus, and Leptospermum (Myrtaceae) in Oceania (Australia including Tasmania and New Zealand) [12,14,19,34,36,101–105]. A color picture of A. niveus (incorrectly named A. eburneus) taken by R.E. Halling in Queensland has recently been published in Mikšík [106].
Austroboletus eburneus is separated from A. subflavidus by the non-areolate pileus surface, elongate fusiform to cylindric subfusiform, narrower basidiospores [(14.5) 15.5–17.5 (19) × 4.4–5.5 µm] with an overall very slightly granular-punctate, rugulose ornamentation and the occurrence in Australia in association with Allocasuarina littoralis (Casuarinaceae) and Eucalyptus spp. (Myrtaceae) [19,34].
The recently described A. roseialbus barely recalls A. subflavidus phenotypically in the general appearance but is easily discriminated on account of the generally smaller size (pileus 3–5 cm broad), viscid-glutinous pileal surface, slimmer (5–7 mm wide) and sticky stipe with cottony surface showing a delicately reticulate pattern, smaller basidiospores (11.2–14 × 6.3–7 µm, Qm = 1.8) with an alveolate-reticulate equatorial ornamentation, smaller basidia (28–35 × 10–14 µm) and the occurrence on the other side of the Pacific Ocean in wet sclerophyll forests under Myrtaceae and Casuarinaceae in New South Wales, Australia [26].
Finally, A. appendiculatus differs from A. subflavidus by the pale brown pileus surface, yolk yellow or golden yellow to pale orange stipe, mild taste, slightly shorter basidiospores (14.2–16.5 × 7.3–9.1 µm, Qm= 1.83), clavate to subclavate or subventricose, larger caulocystidia (40–70 × 11–20 µm) and the occurrence under Shorea robusta (Dipterocarpaceae) in India [49].
Fistulinella gloeocarpa Pegler, Kew Bulletin Additional Series 9: 591. 1983 Figures 4 and 5.
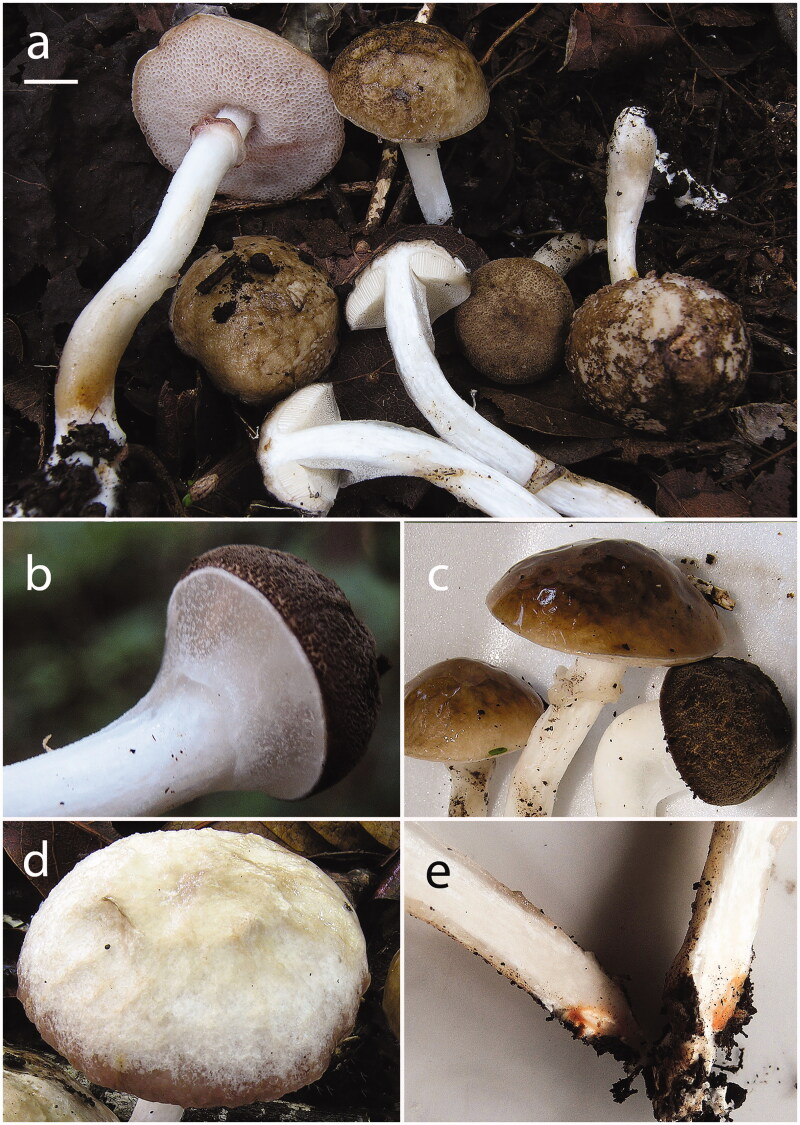
Figure 4.
Fistulinella gloeocarpa basidiomes in habitat. (a) JBSD130769 (ANGE969); (b–d) details of the pileus in various stages of age (b: JBSD130769, ANGE969; c, d: JBSD130770, ANGE970); (e) close up on the pinkish ochraceous spots in the context of the stipe base, JBSD130769 (ANGE969). Scale bars: 1 cm. Photos by C. Angelini.
Figure 5.
Fistulinella gloeocarpa. Micromorphological features; (a) basidiospores; (b) cheilocystidia; (c) pleurocystidia; (d) stipitipellis; (e) basidia; (f) elements of the pileipellis. Scale bars: 10 μm (a–c, e); 20 μm (d, f). Drawings by F. Costanzo.
MYCOBANK MB 124413
Holotype: Lesser Antilles, Martinique, Terreville, on soil in secondary mesophitic forest, 200 m, 10 Oct 1975, J.P. Fiard, 611 A, B (K)
Basidiomes small. Pileus (1.5) 2.0–5.0 (5.5) cm broad, at first hemispherical then persistenly convex and finally broadly pulvinate-flattened, sometimes slightly depressed at center, regularly to hardly unevenly shaped by shallow depressions, moderately fleshy, firm at the beginning but progressively softer with age, flabby in old basidiomes; margin obtuse, steady to faintly wavy-lobed, initially slightly involute soon curved downwards and finally nearly completely plane, not or only a little extending beyond the tubes; surface matt, in the early developmental stages with an innermost gelatinous pellicle underlying a dry, very finely rugulose-granulose outermost layer, later progressively smooth and glabrous, sometimes hammered to delicately wrinkled or coarsely ridged-reticulate (scrobiculate) due to the coagulation of the gelatinous layer, always strongly glutinous with age, irrespective of the weather conditions, not cracked; cuticle decidedly variable in color depending on the weather, ranging from pure white, whitish or pale grayish white to pale brownish gray (White, Pl. LIII; Pale Drab-Gray, Light Grayish Olive, Light Drab, Drab, Pl. XLVI) when rainy but tipically darker, mouse gray or slate gray to brown, dark brown or blackish brown (Mouse Gray, Deep Mouse Gray, Iron Gray, Pl. LI; Sudan Brown, Antique Brown, Argus Brown, Raw Umber, Pl. III; Buckthorn Brown, Dresden Brown, Mummy Brown, Pl. XV; Dark Mouse Gray, Blackish Mouse Gray, Plate LI) when dry, in young specimens always with a narrow white (White, Pl. LIII) marginal rim; not staining on handling or when injured; subcuticular layer white (White, Pl. LIII) to mouse gray or slate gray (Mouse Gray, Deep Mouse Gray, Iron Gray, Plate LI). Tubes at first thin then increasingly broader and decidedly longer than the thickness of the pileus context (up to 1.8 cm long), adnexed to deeply depressed around the stipe apex to nearly free, whitish (White, Pl. LIII) at first then whitish pink to pale flesh-pink, light pinkish lilac (Light Buff, Pl. XV; Seashell Pink, Pale Salmon Color, PL. XIV; Flesh-Pink, Chatenay Pink, Pl. XIII; Pale Purplish Vinaceous, Pale Grayish Vinaceous, Pl. XXXIX; Light Pinkish Lilac, Pl. XXXVII; Brownish Vinaceous, Deep Brownish Vinaceous, Pl. XXXIX) at maturity and further darkening up to cocoa brown (Sayal Brown; Wood Brown, Pl. XL) in old fruiting bodies, unchangeable when cut. Pores initially hidden by a thick, colorless, glutinous veil which soon disrupts revealing the fertile tissue underneath; at the beginning forming a flat surface, later slightly convex to ascendant, at first relatively small then gradually wider (up to 2 mm in diam.), simple, roundish to barely angular at maturity, concolorous with the tubes and not staining on bruising or when injured. Stipe (3.0) 5.0–7.5 (8.0) × (0.4) 0.6–1.1 (1.3) cm, usually longer than or less frequently as long as the pileus diameter at maturity, central to slightly off-center, solid, firm, straight or curved, cylindrical to more frequently sligthly swollen toward the base, ending with a short taproot at the very base; entirely enveloped by a thick, colorless, glutinous membrane which soon disrupts in velar remnants forming an ascending, persistent glutinous annulus located in the upper part of the stipe, eventually becoming cocoa brown (Sayal Brown; Wood Brown, Pl. XL) due to spore discharge; very finely pruinose to smooth and glabrous, devoid of reticulum; white (White, Pl. LIII) throughout but usually with cream yellowish, ochraceous yellow (Martius Yellow, Pl. IV; Naphtalene Yellow, Straw Yellow, Pl. XVI; Primuline Yellow, Pl. XVI) to bright flesh-pink (Light Coral Red, Pl. XIII; Salmon Color, Apricot Buff, Pl. XIV) spots or shades at the stipe base, unchangeable when pressed; basal mycelium white (White, Pl. LIII), rhizomorphs brownish (Mikado Brown, Pl. XXIX). Context firm when young, later soft textured and eventually flabby in the pileus (up to 0.7 cm thick in the central zone), a little more fibrous in the stipe, white (White, Pl. LIII) throughout but in young specimens with a mouse gray or slate gray (Mouse Gray, Deep Mouse Gray, Iron Gray, Plate LI) band just beneath the cuticle, with cream yellowish, ochraceous yellow (Martius Yellow, Pl. IV; Naphtalene Yellow, Straw Yellow, Pl. XVI; Primuline Yellow, Pl. XVI) to bright pinkish (Light Coral Red, Pl. XIII; Salmon Color, Apricot Buff, Pl. XIV) spots or shades at the stipe base; unchangeable when exposed to air; subhymenophoral layer white (White, Pl. LIII); exsiccate pileus dull grayish to brownish (Pale Drab-Gray, Light Grayish Olive, Light Drab, Drab, Pl. XLVI), hymenophore flesh-pink to cocoa brown (Sayal Brown; Wood Brown, Pl. XL), stipe and context whitish to beige (White, Pl. LIII; Maize Yellow, Pl. IV; Marguerite Yellow, Pl. XXX). Odor indistinct. Taste mild. Spore print cocoa brown (Sayal Brown; Wood Brown, Pl. XL). Macrochemical spot-test reactions: 30% KOH: staining wine red everywhere; 25% NH4OH: none.
Basidiospores [70/7/3] (10.3) 12.8 ± 1.00 (16.2) × (4.5) 5.3 ± 0.34 (6.4) µm, Q = (2.00) 2.03–2.75 (2.84), Qm = 2.40 ± 0.17, V = 193 ± 35 µm³, inequilateral, ellipsoid fusiform to fusiform in side view, ellipsoid to ellipsoid fusiform in face view, smooth, apex rounded, with a short apiculus, usually with a shallow suprahilar depression and a slightly pronounced adaxial swelling, moderately thin-walled (0.3–0.5 µm), straw yellow colored in water and 5% KOH, having one, less frequently two or three large oil droplets when mature, rarely pluri-guttulate, inamyloid, strongly cyanophilic and with an ortochromatic reaction. Basidia (21) 23–38 (40) × 10–14 µm (n = 20), subclavate to clavate, moderately thick-walled (0.3–0.8 µm), predominantly 4-spored but also 1-, 2-, or 3-spored, usually bearing relatively short sterigmata (2–5 µm) (sterigmata up to 8 µm long in 1-spored basidia), hyaline to pale yellowish and seldom containing scattered straw-yellow oil guttles in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without basal clamps; basidioles cylindrical-clavate, faintly clavate to clavate, similar in size to basidia. Cheilocystidia (37) 39–56 (60) × 5–9 µm (n = 13), common, moderately slender, projecting straight to sometimes flexuous, irregularly cylindrical or cylindrical fusiform to narrowly fusiform, with rounded to subacute tip, smooth, moderately thick-walled (0.5–1.0 µm), hyaline to pale yellowish in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without epiparietal encrustations. Pleurocystidia (32) 36–58 (65) × (6) 8–13 µm (n = 10), infrequent, color, and chemical reactions similar to but with a different shape, fusiform to ventricose fusiform or lageniform and broader than cheilocystidia. Pseudocystidia not recorded. Pileipellis an ixotrichoderm consisting of interwoven, elongated, frequently branched, filamentous and sinuous to cylindrical, disarticulating and easily detached hyphae heavily embedded in gelatinous matter; terminal elements 22–135 × (4) 5–22 µm, long and slender, filamentous and sinuous to large cylindrical or sausage-shaped, apex rounded-obtuse, thick-walled (up to 2 µm), hyaline to very pale yellowish in water and 5% KOH, golden yellow (inamyloid) in Melzer’s, smooth to sometimes ornamented by a very subtle granular epiparietal encrustation; subterminal elements similar in shape, size and color to terminal elements. Stipitipellis a layer of slender, parallel to loosely intermingled and longitudinally running, smooth-walled, adpressed hyphae, 5–10 µm wide, hyaline to very pale yellowish in water and 5% KOH; the stipe apex covered by a layer 100–150 µm thick of strongly entangled filamentous and sinuous, frequently branched hyphae 2–5 µm broad, having a wall up to 0.3 µm thick, heavily embedded in gelatinous matter, giving rise in the outermost part to disrupted tufts of projecting parallel to subparallel and anticlinally arranged, septate hyphae; terminal elements short cylindrical to irregularly cylindrical or subclavate to peanut-shaped or acorn-shaped, 15–54 × 7–10 µm, occasionally filamentous and up to 80 × 4 µm, apex rounded-obtuse; caulohymenial elements not differentiated or nearly so, caulobasidia infrequent, mostly 1- and 2-spored, 35–45 × 7–10 µm, sterigmata up to 7 µm long (n = 6), caulocystidia not observed. Lateral stipe stratum absent. Stipe trama composed of confusedly and densely arranged, subparallel to moderately interwoven, filamentous, smooth, inamyloid hyphae, 4–22 µm broad. Hymenophoral trama bilateral divergent of the “Boletus-type”, with slightly to strongly divergent, recurved-arcuate and loosely arranged, not-branched, distantly septate and generally restricted at septa, gelatinous hyphae (lateral strata hyphae in transversal section not touching each other, (4) 5–12 (15) µm apart, 5–12 µm broad), hyaline to very pale yellowish in water and 5% KOH, inamyloid in Melzer’s; lateral strata (20) 30–80 (90) µm thick, mediostratum (10) 20–40 (50) µm thick, axially arranged, consisting of a tightly adpressed, non-gelatinous bundle of hyphae, 2–8 µm broad, more frequently septate; in Congo Red the mediostratum is darker than the lateral strata. Oleipherous hyphae scattered although more frequently observed in the basal stipe trama, golden yellow to brownish in 5% KOH and Melzer’s. Clamp connections absent in all tissues. Ontogenetic development probably hemiangiocarpic (monovelangiocarpic) due to the presence of a thick, glutinous and colorless, universal veil enveloping the entire basidiomes.
Edibility unknown.
Ecology and phenology: gregarious, growing on limestone among litter in a seasonally dry and moist anthropized lowland mixed stand under a large array of neotropical broadleaved trees including Coccoloba diversifolia (Polygonaceae) and (in Martinique) perhaps also with Haematoxylum sp. (Caesalpinoideae), which represent its possible ECM host trees. See Parra et al. [107] for further details on lowland vegetation in the Dominican Republic. Apparently localized in the Dominican Republic. August to March.
Known distribution: to date only known from both the Lesser and Greater Antilles islands of the Caribbean (Martinique, the Bahamas and the Dominican Republic) and in all probability in south-eastern USA (Florida) in tropical environment (see below).
Examined material: DOMINICAN REPUBLIC, Municipality of Sosúa, Puerto Plata Province, loc. cemetery, three km away from the seaside, 19°44′40″N 70°32′21″W, 100 m, 01 Dec 2017, several specimens in all developmental stages, C. Angelini (JBSD130769, ANGE969, and MG777); same loc., 02 Dec 2017, several specimens in all developmental stages, C. Angelini (JBSD130770, ANGE970, and MG778); same loc. 28 Mar 2020, a single mature specimen, C. Angelini (ANGE1147).
Comments: Macro-morphologically, anatomically, and ecologically, samples in this study almost perfectly match the description of Fistulinella gloeocarpa described by Pegler [55] from Martinique (Lesser Antilles) based on material mostly collected by J.P. Fiard and by Pegler. This species can be recognized on account of the following combination of characters: small basidiomes (pileus up to 5.5 cm diam.), pileus surface at first dry and rugulose-granulose to progressively smooth, sometimes hammered to finely wrinkled-reticulate or scrobiculate and then strongly glutinous with age, ranging from whitish, grayish or grayish brown to dark brown or blackish brown, white to pinkish hymenophore covered by a thick, colorless and glutinous veil in early developmental stages, smooth, glutinous, white stipe usually showing yellowish ochraceous shades or patches at the base and with a persistent glutinous annulus at maturity, white context and basal mycelium, unchanging tissues on bruising or injury, mild taste, cocoa brown spore deposit, reddish staining reaction with KOH on all tissues, ellipsoid-fusiform, smooth, strongly cyanophilic basidiospores, ixotrichoderm pileipellis consisting of filamentous to cylindrical hyphae and the occurrence in low-elevation neotropical environments in alleged association with Coccoloba diversifolia (this plant was found at the collection sites in both Martinique and the Dominican Republic) and Haematoxylum sp. ([55]; this study).
A considerable amount of specimens collected in the field in the Dominican Replublic has given us the opportunity to recognize reliable discriminating features for separating F. gloeocarpa from a number of morphological lookalikes occurring in Central and northern South America, such as F. jamaicensis (Murrill) Singer, F. venezuelae (Singer & Digilio) Singer, F. mexicana Guzmán, F. campinaranae Singer, F. cinereoalba Fulgenzi & T.W. Henkel and F. ruschii A.C. Magnago.
Fistulinella jamaicensis is separated from F. gloeocarpa by its tiny basidiomes (pileus up to 1.8 cm diam., stipe 3 cm long, 3.5 mm wide), somewhat areolate pileus surface, smaller basidiospores [(9.5) 10–11 (12) × (4) 4.8–5 (5.2) µm, Qm = 2.0], shorter hymenial cystidia (20–30 × 10–12 µm) with apical ampullaceous neck and apparently an absence of veils [20,22,108,109]. Lewis and Cibula [110] and more recently Bessette et al. [95] provided a re-description of F. jamaicensis from southern USA emphasizing characters such as a pileus up to 4.5 cm diam., pinkish or brownish pink than grayish to grayish brown pileus with amber-yellow spots in age, stipe often with brownish scales and spores 8.5–14.5 × 4.5–6.5 µm. Given the several morphological discrepancies when a comparison is made with the original description by Murrill [108], we suspect it does not represent the same taxon.
Fistulinella venezuelae differs by the whitish to yellowish pileus at the margin with yellow ochraceous to tawny center, ochraceous-ferruginous tints in the upper part of the stipe at maturity and pale yellowish to brownish gray mealy punctuations in the lower portion, white context with a pale ochraceous peripheral zone, absence of velar covering, weakly bitterish taste, elongate fusiform-cylindrical, much longer basidiospores [(12) 14.5–21.5 × 4.5–6 (6.5) µm, Qm= 3.2], generally longer hymenial cystidia (up to 93 µm long) usually exhibiting a long and slender neck, a cutis pileipellis with markedly narrower filamentous hyphae [(2) 3.5–10.5 µm wide] and growth in mountain environment in doubtful association with Alnus acuminata in Venezuela or in lowland vegetation in the Lesser Antilles (Martinique, Dominica) [14,20,51,55,111–113]. Additional collections of F. venezuelae have been made in Puerto Rico, Virgin Islands, and French Guyana (MycoPortal).
Guzmán described F. mexicana from evergreen lowland cloud forests in the Yucatan peninsula, southern-eastern Mexico [52] in putative association with Coccoloba spp. [114]. This species is distinguished from F. gloeocarpa by the yellowish brown or grayish, irregularly areolate pileus surface, presence of a colorless mucilaginous volva at the stipe base, slightly shorter basidiospores (8.1–12.2 × 4.1–5.9 µm), smaller, clavate pleurocystidia (24–43 × 5–8 µm), cheilocystidia none, cylindrical-globose caulocystidia (35–48 × 12–16 µm), narrower pileipellis hyphae (2.5–6.5 µm wide) and sometimes with an apparently lignicolous growth [20,30,52,114,115]. This species has most recently check listed for the Mexican state of Quintana Roo by de la Fuente et al. [114].
Even if outwardly very similar, F. campinaranae and its var. scrobiculata Singer can be discriminated from F. gloeocarpa by the presence of a membranous but fugacious whitish ring on the stipe, slightly narrower, dextrinoid basidiospores [(11.5) 12–15 (18) × (3) 4–5 (6) µm, Qm = 3.3], narrower pileipellis hyphae (3.4–10 µm broad), narrower hyphae of lateral stratum (2–6 µm wide) in the hymenophoral trama and the occurrence on rotting wood and decayed stumps or less frequently on humus-sandy soil in Brazilian Amazonian caatinga and campinarana vegetation, in lowland Colombian rainforests dominated by Pseudomonotes (Dipterocarpaceae) and along the Brazilian coastal Atlantic Forest (Bahia) under leguminous trees (Fabaceae) [20,31,42,57,116]. This species is also separated from F. gloeocarpa based on molecular inference [42]. Regrettably, efforts for extracting DNA from either the holotype collection (not located at INPA) and paratype samples resulted unsuccessful [42].
Fistulinella gloeocarpa and F. cinereoalba are two look-alike species and phylogenetically most closely related to each other, being sister species in the molecular analysis (Figure 1). The latter species, however, can be unraveled based on the stipe base devoid of yellowish ochraceous spots, very finely squamulose stipe surface, hymenophore and stipe turning brownish when injured, decidedly longer and slightly narrower, variably dextrinoid basidiospores [12.4–19.8 (24.8) × 3.7–4.9 (6) µm, Qm= 3], aciculate to cylindrical, narrower pleurocystidia (37–61 × 3.7–6.2 µm), narrower pileipellis hyphae (2.4–7 µm broad), narrower hyphae of lateral stratum (2–6 µm wide) in the hymenophoral trama and the occurrence in Guyana in association with Dicymbe corymbosa (Fabaceae subfamily Caesalpinioideae) and along the coastal Atlantic Forest in Brazil (Bahia) [27,116–118]. Magnago [118] reports much broader pleurocystidia (43–76 × 10–17 µm) for the Brazilian collections.
In some regards, F. gloeocarpa is also similar to F. ruschii, however, corroborative features for distinguishing the latter species include the tomentose and mostly dry, chestnut brown to orange-brown pileus surface, cream pinkish stipe, NH4OH staining reddish orange and yellow on pileus and stipe, respectively, longer and narrower basidiospores [14–18 (22) × 4–5 µm, Qm = 3.4], presence of broadly cylindrical, multiseptate pleurocystidia, narrower pileipellis hyphae (4–11 µm broad), narrower hyphae of lateral stratum (4–7 µm wide) in the hymenophoral trama and the occurrence along the Brazilian coastal Atlantic Forest under caesalpinoid legumes (Fabaceae) and the Virgin Islands of the Caribbean ([118] as “F. rhytidocystidiata Magnago & M.A. Neves ad int.”, [42]; this study). Based on morphological resemblance and preliminary phylogenetic inference (Figure 1) it appears quite possible that F. ruschii and F. venezuelae might represent the same taxon (collection TJB-8329 was formerly identified by T.J. Baroni as F. venezuelae). Should this conspecificity be confirmed, F. venezuelae would have priority over F. ruschii having been described previously than the latter species but further studies are needed to elucidate their taxonomic relationships.
4. Discussion
The bulk of Austroboletus based on the data mining and phylogenetic inference (Figure 1) indicates there is a core for the genus, including the type species (clade I). A very small number of species (including A. subflavidus) attributed to Austroboletus that fall outside the core (clades I and II), suggesting polyphyly, need a closer look and further analyses. Accordingly, the disposition of A. subflavidus ultimately results uncertain, nonetheless we feel that any transfer to either a new genus or a new subgenus (corresponding to clade II) would at present be premature.
Macro- and micro-morphological features of Dominican samples of A. subflavidus studied herein consistently match those retrievable in the aforementioned available literature but they display, as already pointed out by Ortiz-Santana et al. [29] based on Dominican and Belizean material, generally smaller dimensions and perhaps minor anatomical differences with regard to the North American populations, probably due to geographic distance and different hosts or dissimilar climatic conditions.
Concerning biogeography, A. subflavidus shows a broad distribution, spanning from warm temperate to subtropical Atlantic regions of eastern, south-eastern USA and Mexico south to the neotropical countries of continental and insular Central America [12,20,25,29,96] and northern South America [31]. All reports of A. subflavidus from outside its natural distribution range in the western hemisphere (see, among others, [119–121]) should be carefully re-assessed.
As far as the genus Fistulinella is concerned, since it was first described from Martinique [55] F. gloeocarpa has not anymore been recollected elsewhere, albeit Vasco-Palacios et al. [31] suggested a possible occurrence in lowland Pseudomonotes (Dipterocarpaceae) forests in Colombian Amazonia but did not provide any convincing evidence. Accordingly, the present account is the only documented record of Fistulinella gloeocarpa from outside the Lesser Antilles and the first from the Dominican Republic. Indeed, genetic material generated in this study match with a sequence deposited in GenBank (GQ981503, collection KM162946) and obtained by D.J. Lodge from the Bahamas islands, further widening the distribution range of F. gloeocarpa. Moreover, three additional molecularly unconfirmed records of this species have been spotted under Coccoloba diversifolia in Florida by J. Bolin (JAB211) and by Alan R. Franck (4662) in 2018 and published online in the mycological website Mushrooms Observer (https://mushroomobserver.org/observer/show_observation/359000). Unfortunately, it has not been possible to either reexamine the holotype material or paratype collections made by Pegler in Martinique in the ‘70 s preserved at the Royal Botanic Gardens Kew (K). On the other hand, no major morphological discrepancies can be observed when comparing the Dominican collections with the original description [55], despite its evident morphological variability. As a matter of fact, depending on the weather conditions and developmental stages, basidiomes of F. gloeocarpa may be extremely mutable concerning their morphological appearance. The most variable characters are the texture and color of the pileal surface; the surface is initially dark colored and finely rugulose-granulose but tends to become much paler, smooth, glutinous, and often typically rugulose-scrobiculate. Neither in the original diagnosis nor in the comments of F. gloeocarpa mention is made about the presence of yellowish ochraceous spots at the stipe base [55]. However, a yellowish patch is clearly visible in one of the two color photographs (Pl. 19E-F) accompanying the original description, indicating that this chromatic trait was simply overlooked by Pegler. Likewise Pegler [55] did not report the presence of a ring on the stipe of F. gloeocarpa but it might have been removed accidentally by handling or simply gone unnoticed. However, the presence of a thick, persistent glutinous annulus obviously reflects an angiocarpic ontogenetic development and may determine, in addition to the mucilaginous volva reported for F. mexicana Guzmán (see below), an emendation of the diagnostic traits of Fistulinella, which was thought to be devoid of veils in all its representatives [16,32,55]. It would be advisable, however, to propose such an emendation only when the taxonomic limits of Fistulinella are better clarified.
In spite of the fact that morphological differences seem to justify specific separation of F. gloeocapa from the several closely allied species occurring in the same geographic macro-region, some of them (F. jamaicensis, F. mexicana, and F. venezuelae) are still lacking molecular confirmation and further research will be required to confirm their autonomous taxonomic status and mutual phylogenetic relationships. Especially F. jamaicensis and F. mexicana might finally turn out to be conspecific with F. gloeocarpa given morphological affinities and geographic proximity, but until sequenced material from Jamaica and Mexico is not available for comparison, it will be advisable to maintain these taxa as separate entities.
Acknowledgments
MG is indebted to M. Atzeni (Anguillara Sabazia, Italy) for the logistic help. D.J. Lodge (Athens, GA) is warmly acknowledged for the information provided on the occurrence of F. gloeocarpa and related taxa in the Bahamas. T.J. Baroni (State University of New York, Cortland) kindly supported us with an unpublished sequence of F. ruschii from the Virgin Islands, CA wishes to thank Ricardo G. García, Francisco Jiménez, Brígido Peguero (Jardín Botánico Nacional Dr. Rafael M. Moscoso, Santo Domingo, Dominican Republic) for their interest and encouragement in studying fungi of the Dominican Republic and for their active cooperation in providing herbarium material preserved in their institution. Thanks are also given to R.E. Halling (New York Botanical Garden, New York, NY) and to the anonymous reviewers for their constructive comments and valuable suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Binder M. Zur molekularen Systematische der Boletales: Boletineae und clerodermatineae subordo nov [PhD dissertation]. Regensburg: Nat Fak III-Biol Vorkl Med, Universität Regensburg; 1999. [Google Scholar]
- 2.Binder M, Hibbett DS.. Molecular systematics and biological diversification of Boletales. Mycologia. 2006;98(6):971–981. [DOI] [PubMed] [Google Scholar]
- 3.Drehmel D, James T, Vilgalys R.. Molecular phylogeny and biodiversity of the boletes. Fungi. 2008;1(4):17–23. [Google Scholar]
- 4.Halling RE, Fechner N, Nuhn M, et al. . Evolutionary relationships of Heimioporus and Boletellus (Boletales), with an emphasis on Australian taxa including new species and new combinations in Aureoboletus. Aust Syst Bot. 2015;28(1):1–22. [Google Scholar]
- 5.Kuo M, Ortiz-Santana B.. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia. 2020;112(1):197–211. [DOI] [PubMed] [Google Scholar]
- 6.Nuhn ME, Binder M, Taylor AFS, et al. . Phylogenetic overview of the Boletineae. Fungal Biol. 2013;117(7–8):479–511. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Feng B, Xu JP, et al. . Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014;69(1):93–115. [Google Scholar]
- 8.Wu G, Li YC, Zhu XT, et al. . One hundred noteworthy boletes from China. Fungal Divers. 2016;81(1):25–188. [Google Scholar]
- 9.Corner EJH. Boletus in Malaysia. Singapore: Government Printing Office; 1972. [Google Scholar]
- 10.Horak E. Boletellus and Porphyrellus in Papua New Guinea. Kew Bull. 1977;31(3):645–652. [Google Scholar]
- 11.Wolfe CB Jr, Petersen RH.. Taxonomy and nomenclature of the supraspecific taxa of Porphyrellus. Mycotaxon. 1978;7:152–162. [Google Scholar]
- 12.Wolfe CB., Jr.Austroboletus and Tylopilus subg. Porphyrellus, with emphasis on North American taxa Bibliotheca Mycologica. 69 Berlin Stuttgart: Cramer; 1980. [Google Scholar]
- 13.Corner EJH. Boletus longipes Massee, a critical Malaysian species. Garden’s Bulletin, Singapore. 1980;33(2):290–296. [Google Scholar]
- 14.Pegler DN, Young TWK.. A natural arrangement of the Boletales, with reference to spore morphology. Trans Br Mycol Soc. 1981;76(1):103–146. [Google Scholar]
- 15.Singer R. Notes on bolete taxonomy – III. Persoonia. 1981;11(3):269–302. [Google Scholar]
- 16.Singer R. The Agaricales in modern taxonomy, 4th ed Koenigstein: Koeltz Scientific Books; 1986. [Google Scholar]
- 17.Horak E. Supplementary remarks to Austroboletus (Corner) Wolfe (Boletaceae). Sydowia. 1980;33:71–87. [Google Scholar]
- 18.Horak E. Mycogeography in the South Pacific region: Agaricales, Boletales. Austral J Bot. 1983;10:1–41. [Google Scholar]
- 19.Watling R, Gregory NM.. Observations on the boletes of the Cooloola Sandmass, Queensland and notes on their distributions in Australia. Proc Roy Soc Queensl. 1986;97:97–128. [Google Scholar]
- 20.Singer R, Araujo I, Ivory MH.. The ectotrophically mycorrizal fungi of the neotropical lowlands, especially central Amazonia (litter decomposition and ectomycorrhiza in Amazonian forests 2. Beihefte Zur Nova Hedwigia. 1983;77:1–352. [Google Scholar]
- 21.He MQ, Zhao RL, Hyde KD, et al. . Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019;99(1):105–367. [Google Scholar]
- 22.Singer R. The Boletineae of Florida with notes on extralimital species. II. The Boletaceae (Gyroporoideae). Farlowia. 1945b;2(2):223–303. [Google Scholar]
- 23.Smith AH, Thiers HD.. The boletes of Michigan. Ann Arbor (MI): University of Michigan Press; 1971. [Google Scholar]
- 24.Bessette AE, Roody WC, Bessette AR.. North American boletes. A color guide to the fleshy pored mushrooms. Syracuse (NY): Syracuse University Press; 2000. [Google Scholar]
- 25.Bessette AE, Roody WC, Bessette AR.. Boletes of Eastern North America. Syracuse (NY): Syracuse University Press; 2016. [Google Scholar]
- 26.Fechner N, Bonito G, Bougher NL, et al. . New species of Austroboletus (Boletaceae) in Australia. Mycol Progress. 2017;16(8):769–775. [Google Scholar]
- 27.Fulgenzi TD, Halling RE, Henkel TW.. Fistulinella cinereoalba sp. nov. and new distribution records for Austroboletus from Guyana. Mycologia. 2010;102(1):224–232. [DOI] [PubMed] [Google Scholar]
- 28.Halling RE, Osmundson TW, Neves MA.. Austroboletus mutabilis sp. nov. from northern Queensland. Muelleria. 2006;24:31–36. [Google Scholar]
- 29.Ortiz-Santana B, Lodge DJ, Baroni TJ, et al. . Boletes from Belize and the Dominican Republic. Fungal Divers. 2007;27:247–416. [Google Scholar]
- 30.Singer R, García J, Gómez LD.. The Boletineae of Mexico and Central America III. Beihefte Zur Nova Hedwigia. 1991;102:1–99. [Google Scholar]
- 31.Vasco-Palacios AM, Lopez-Quintero CA, Franco-Molano AE, et al. . Austroboletus amazonicus sp. nov. and Fistulinella campinaranae var. scrobiculata, two commonly occurring boletes from a forest dominated by Pseudomonotes tropenbosii (Dipterocarpaceae) in Colombian Amazonia. Mycologia. 2014;106(5):1004–1014. [DOI] [PubMed] [Google Scholar]
- 32.Watling R. A manual and source book on the boletes and their allies Synopsis Fungorum. 24 Oslo: Fungiflora; 2008. [Google Scholar]
- 33.Watling R, de Meijer AR.. Macromycetes from the State of Paraná, Brazil 5. Poroid and lamellate boletes. Edinburgh J Bot. 1997;54(2):231–251. [Google Scholar]
- 34.Watling R, Li TH.. Australian boletes. A preliminary survey. Edinburgh: Royal Botanic Garden Edinburgh; 1999. [Google Scholar]
- 35.Ying JZ. Notes on the genus Austroboletus in China. Agarica. 1985;6(12):80–89. [Google Scholar]
- 36.McNabb RFR. The Strobilomycetaceae of New Zealand. N Z J Bot. 1967;5(4):532–547. [Google Scholar]
- 37.Crous PW, Luangsa-Ard JJ, Wingfield MJ, et al. . Fungal planet description sheets: 785–867. Persoonia. 2018;41:238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crous PW, Wingfield MJ, Lombard L, et al. . Fungal planet description sheets: 951–1041. Persoonia. 2019;43:223–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farid A, Gelardi M, Angelini C, et al. . Phylloporus and Phylloboletellus are no longer alone: Phylloporopsis gen. nov. (Boletaceae), a new smooth-spored lamellate genus to accommodate the American species Phylloporus boletinoides. Fungal Syst Evol. 2018;2:341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henkel TW, Obase K, Husbands D, et al. . New Boletaceae taxa from Guyana: Binderoboletus segoi gen. and sp. nov., Guyanaporus albipodus gen. and sp. nov., Singerocomus rubriflavus gen. and sp. nov., and a new combination for Xerocomus inundabilis. Mycologia. 2016;108(1):157–173. [DOI] [PubMed] [Google Scholar]
- 41.Khmelnitsky O, Davoodian N, Singh P, et al. . Ionosporus: a new genus for Boletus longipes (Boletaceae), with a new species, I. australis, from Australia. Mycol Progress. 2019;18(3):439–451. [Google Scholar]
- 42.Magnago AC, Neves MA, da Silveira BRM.. Fistulinella ruschii, sp. nov., and a new record of Fistulinella campinaranae var. scrobiculata for the Atlantic Forest, Brazil. Mycologia. 2017;109(6):1003–1013. [DOI] [PubMed] [Google Scholar]
- 43.Orihara T, Smith ME.. Unique phylogenetic position of the African truffle-like fungus, Octaviania ivoryana (Boletaceae, Boletales), and the proposal of a new genus, Afrocastellanoa. Mycologia. 2017;109(2):323–332. [DOI] [PubMed] [Google Scholar]
- 44.Smith ME, Amses KR, Elliott TF, et al. . New sequestrate fungi from Guyana: Jimtrappea guyanensis gen. sp. nov., Castellanea pakaraimophila gen. sp. nov., and Costatisporus cyanescens gen. sp. nov. (Boletaceae, Boletales. IMA Fungus. 2015;6(2):297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sulzbacher MA, Orihara T, Grebenc T, et al. . Longistriata flava (Boletaceae, Basidiomycota) – a new monotypic sequestrate genus and species from Brazilian Atlantic Forest. MycoKeys. 2020;62:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao K, Wu G, Yang ZL.. A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa. 2014;188(2):61–77. [Google Scholar]
- 47.Zhao K, Wu G, Halling RE, et al. . Three new combinations of Butyriboletus (Boletaceae). Phytotaxa. 2015;234(1):51–62. [Google Scholar]
- 48.Das K, Dentinger BTM.. Austroboletus olivaceoglutinosus, a new mushroom species from Sikkim, India with a distinctive green, glutinous pileus. Kew Bull. 2015;70(1):15. [Google Scholar]
- 49.Tibpromma S, Hyde KD, Jeewon R, et al. . Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017;83(1):1–261. [Google Scholar]
- 50.Hennings P. Beiträge zur Flora von Afrika. XXI. Fungi Camerunenses Novi. III. Botanische Jahrbücher Fur Systematik. 1901;30:39–57. [Google Scholar]
- 51.Singer R. Strobilomycetaceae (Basidiomycetes). Flora Neotropica 5. New York (NY): Hafner; 1970. [Google Scholar]
- 52.Guzmán G. El género Fistulinella Henn. (= Ixechinus R. Heim) y las relaciones florísticas entre México y Africa. Boletín de la Sociedad Mexicana de Micología. 1974;8:53–63. [Google Scholar]
- 53.Horak E. Synopsis generum Agaricalium (Die Gattungstypen der Agaricales). Waber-Bern: Kommissions verlag Druckerei Büchler; 1968. [Google Scholar]
- 54.Horak E. Revision of Malaysian species of Boletales s. l. (Basidiomycota) described by E. J. H. Corner (1972, 1974). Kuala Lumpur: Malayan Forest Records; 2011. [Google Scholar]
- 55.Pegler DN. Agaric flora of the Lesser Antilles Kew Bulletin Additional Series. London: HMSO; 1983. [Google Scholar]
- 56.Redeuilh GL, Soop K.. Nomenclature et taxinomie des genres affines à Fistulinella (Boletaceae) et Fistulinella lutea sp.nov. de Nouvelle Zélande. Bulletin de la Société Mycologique de France. 2006;122(4):291–304. [Google Scholar]
- 57.Singer R. Notes on bolete taxonomy – II. Persoonia. 1978;9(4):421–438. [Google Scholar]
- 58.Watling R. Australian boletes: their diversity and possible origins. Aust Syst Bot. 2001;14(3):407–416. [Google Scholar]
- 59.Thiers B. Index Herbariorum: a global directory of public herbaria and associated staff. New York botanical garden’s virtual herbarium. http://sweetgum.nybg.org/ih/. Accessed 10 July 2020.
- 60.Ridgway R. Color standards and color nomenclature. Washington (DC): Self-published; 1912. [Google Scholar]
- 61.Roy M, Schimann H, Braga-Neto R, et al. Diversity and distribution of ectomycorrhizal fungi from Amazonian lowland white-sand forests in Brazil and French Guiana. Biotropica. 2016;48(1):90–100. [Google Scholar]
- 62.Orihara T, Lebel T, Ge Z-W, et al. . Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite-like insertions for molecular identification. Persoonia. 2016;37:173–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vadthanarat S, Lumyong S, Raspé O.. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys. 2019;54:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hosen MI, Feng B, Wu G, et al. . Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Divers. 2013;58(1):215–226. [Google Scholar]
- 65.Young AP, Evans RC, Newell R, et al. . Development of a DNA barcoding protocol for fungal specimens from the E.C. Smith Herbarium (ACAD). Northeast Nat. 2019;26(3):465–483. [Google Scholar]
- 66.Porter TM, Skillman JE, Moncalvo JM.. Fruiting body and soil rDNA sampling detects complementary assemblage of Agaricomycotina (Basidiomycota, Fungi) in a hemlock-dominated forest plot in southern Ontario. Mol Ecol. 2008;17(13):3037–3050. [DOI] [PubMed] [Google Scholar]
- 67.Halling RE, Nuhn M, Osmundson T, et al. . Affinities of the Boletus chromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Aust. Systematic Bot. 2012;25(6):418–431. [Google Scholar]
- 68.Smith ME, Henkel TW, Aime CM, et al. . Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a neotropical rainforest. New Phytol. 2011;192(3):699–712. [DOI] [PubMed] [Google Scholar]
- 69.Orihara T, Smith ME, Shimomura N, et al. . Diversity and systematics of the sequestrate genus Octaviania in Japan: two new subgenera and eleven new species. Persoonia. 2012;28:85–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halling RE, Baroni TJ, Binder M.. A new genus of Boletaceae from eastern North America. Mycologia. 2007;99(2):310–316. [DOI] [PubMed] [Google Scholar]
- 71.Zeng NK, Su MS, Liang ZQ, et al. . A geographical extension of the North American genus Bothia (Boletaceae, Boletales) to East Asia with a new species B. fujianensis from China. Mycol Progress. 2015;14(1):6. [Google Scholar]
- 72.Binder M, Bresinsky A.. Retiboletus, a new genus for a species-complex in the Boletaceae producing retipolides. Feddes Repert. 2002;113(1–2):30–40. [Google Scholar]
- 73.Trappe JM, Castellano MA, Halling RE, et al. . Australasian sequestrate fungi 18: Solioccasus polychromus gen. & sp. nov., a richly colored, tropical to subtropical, hypogeous fungus. Mycologia. 2013;105(4):888–895. [DOI] [PubMed] [Google Scholar]
- 74.Li YC, Ortiz-Santana B, Zeng NK, et al. . Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia. 2014;106(2):291–306. [DOI] [PubMed] [Google Scholar]
- 75.Vizzini A, Consiglio G, Marchetti M, et al. . Insights into the Tricholomatineae (Agaricales, Agaricomycetes): a new arrangement of Biannulariaceae and Callistosporium, Callistosporiaceae fam. nov., Xerophorus stat. nov., and Pleurocollybia incorporated into Callistosporium. Fungal Diversity. 2020;101(1):211–259. [Google Scholar]
- 76.Gardes M, Bruns TD.. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118. [DOI] [PubMed] [Google Scholar]
- 77.White TJ, Bruns TD, Lee S, et al. . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In Innis MA, Gelfand DH, Sninsky J, et al., editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. pp. 315–322. [Google Scholar]
- 78.Rehner SA, Samuels GJ.. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res. 1994;98(6):625–634. [Google Scholar]
- 79.Vilgalys R, Hester M.. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172(8):4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu YJ, Whelen S, Hall BD.. Phylogenetic relationships among Ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol. 1999;16(12):1799–1808. [DOI] [PubMed] [Google Scholar]
- 81.Matheny PB, Wang Z, Binder M, et al. . Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol Phylogenet Evol. 2007;43(2):430–451. [DOI] [PubMed] [Google Scholar]
- 82.Kearse M, Moir R, Wilson A, et al. . Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altschul SF, Gish W, Miller W, et al. . Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. [DOI] [PubMed] [Google Scholar]
- 84.Katoh K, Misawa K, Kuma K, et al. . MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darriba D, Taboada GL, Doallo R, et al. . jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Binder M, Fischer M.. Molekularbiologische Charakterisierung der Gattungen Boletellus und Xerocomus: Xerocomus pruinatus (Fr. & Hoek) Quel. und verwandte Arten. Bollettino Del Gruppo Micologico G Bresadola. 1997;40(2–3):79–90. [Google Scholar]
- 87.Ronquist F, Teslenko M, van der Mark P, et al. . MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. [DOI] [PubMed] [Google Scholar]
- 89.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783–791. [DOI] [PubMed] [Google Scholar]
- 90.Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans; 2010. p. 1–8. [Google Scholar]
- 91.Halling RE. An annotated index to species and infraspecific taxa of Agaricales and Boletales described by William A. Murrill. Mem New York Bot Garden. 1986;40:1–120. [Google Scholar]
- 92.Murrill WA. New boletes. Mycologia. 1938;30(5):520–525. [Google Scholar]
- 93.Snell WH. The genera of the Boletaceae. Mycologia. 1941;33(4):415–423. [Google Scholar]
- 94.Singer R. The Boletineae of Florida with notes on extralimital species. I. The Strobilomycetaceae. Farlowia. 1945;2(1):97–141. [Google Scholar]
- 95.Bessette AE, Bessette AR, Lewis DP.. Mushrooms of the Gulf Coast States: A field guide to Texas, Louisiana, Mississippi, Alabama, and Florida. Austin (TX): University of Texas Press; 2019. [Google Scholar]
- 96.Both EE. The boletes of North America. A compendium. Buffalo (NY): Buffalo Museum of Science; 1993. [Google Scholar]
- 97.Grand LF, Moore RT.. Scanning electron microscopy of basidiospores of species of Strobilomycetaceae. Can J Bot. 1971;49(8):1259–1261. [Google Scholar]
- 98.Thiers HD. The bolete flora of the Gulf coastal plain. I. The Strobilomycetaceae. J Elisha Mitchell Sci Soc. 1963;79:32–41. [Google Scholar]
- 99.Weber NS, Smith AH.. A field guide to southern mushrooms. Ann Arbor (MI): University of Michigan Press; 1985. [Google Scholar]
- 100.Trappe JM. Fungus associates of ectotrophic mycorrhizae. Bot. Rev. 1962;28(4):538–606. [Google Scholar]
- 101.Gates G, Ratkowsky D.. A field guide to Tasmanian fungi. 3rd ed Hobart: Tasmanian Field Naturalists Club; 2018. [Google Scholar]
- 102.McKenzie EHC, Buchanan PK, Johnston PR.. Checklist of fungi on Nothofagus species in New Zealand. N Z J Bot. 2000;38(4):635–720. [Google Scholar]
- 103.Segedin BP. An annotated checklist of agarics and boleti recorded from New Zealand. N Z J Bot. 1987;25(2):185–215. [Google Scholar]
- 104.Stevenson G. The Agaricales of New Zealand. I. Boletaceae and Strobilomycetaceae. Kew Bull. 1962;15(3):381–385. [Google Scholar]
- 105.Taylor GM. Mushrooms and toadstools in New Zealand. Mobil New Zealand Nature Series. Wellington: AH & AW Reed; 1981. [Google Scholar]
- 106.Mikšík M. Hřibovité Houby Evropy. Praha: Vydalo nakladatelství Svojtka & Co; 2017. [Google Scholar]
- 107.Parra AL, Angelini C, Ortiz-Santana B, et al. . The genus Agaricus in the Caribbean. Nine new taxa mostly based on collections from the Dominican Republic. Phytotaxa. 2018;345(3):219–271. [Google Scholar]
- 108.Murrill WA. A new Boletus from Jamaica. Mycologia. 1910;2(6):305–305. [Google Scholar]
- 109.Saccardo PA, Trotter A. Supplementum Universale, Pars VIII. Sylloge Fungorum omnium hucusque cognitorum 21. Patavia; 1912. [Google Scholar]
- 110.Lewis DP, Cibula WG.. Studies on Gulf Coast Agarics (Basidiomycota: Agaricaceae): notes on some interesting and rare species. Texas J Sci. 2000;52:65–78. [Google Scholar]
- 111.Dennis RWG. Fungus flora of Venezuela and adjacent countries. Kew Bulletin Additional Series 3. Kew: Royal Botanic Garden; 1970. [Google Scholar]
- 112.Singer R, Digilio APL.. Las boletáceas de Sudamérica tropical. Lilloa. 1960;30:141–164. [Google Scholar]
- 113.Wolfe CB., Jr. Mucilopilus, a new genus of the Boletaceae, with emphasis on North American taxa. Mycotaxon. 1979;10(1):116–132. [Google Scholar]
- 114.de la Fuente JI, García-Jiménez J, López CY, et al. . An annotated checklist of the macrofungi (Ascomycota, Basidiomycota, and Glomeromycota) from Quintana Roo, Mexico. CheckList. 2020;16(3):627–648. [Google Scholar]
- 115.Wolfe CB., Jr A taxonomic evaluation of the generic status of Ixechinus and Mucilopilus (Ixechineae, Boletaceae). Mycologia. 1982;74(1):36–43. [Google Scholar]
- 116.Putzke J, Putzke MTL.. Cogumelos (fungos Agaricales) no Brasil - Ordens Boletales (Boletaceae e Paxillaceae), Polyporales (Polyporaceae/Lentinaceae), Russulales (Russulaceae) e Agaricales (Cortinariaceae, Inocybaceae, Pluteaceae e Strophariaceae). São Gabriel. 2019;2. [Google Scholar]
- 117.Fulgenzi TD. Systematics of Boletaceae from the Guiana Shield [thesis]. Arcata (CA): Humboldt State University; 2009. [Google Scholar]
- 118.Magnago AC. Taxonomia e sistemática de Boletaceae (Boletales) para o Brasil [Dissertação Pós-Graduação]. Florianópolis: Universidade Federal de Santa Catarina; 2014. [Google Scholar]
- 119.May TW, Wood AE.. Catalogue and bibliography of Australian macrofungi 1. Basidiomycota In: Fungi of Australia, vol. 2A Canberra: CSIRO Publishing; 1997. [Google Scholar]
- 120.Thongklam S. Diversity of boletes in some national parks of upper northern Thailand [PhD dissertation]. Chiang Mai: Chiang Mai University; 2008. [Google Scholar]
- 121.Watling R, Hollands R.. Boletes from Sarawak. Notes from the Royal Botanic Garden, Edinburgh. 1990;46(3):405–422. [Google Scholar]