Abstract
Background
Although much is still unknown about the full effects of COVID-19, literature from the early stages of the COVID-19 pandemic (spring and summer 2020) supports a postviral immunologic reaction resulting in a multisystem inflammatory syndrome in children (MIS-C). The purpose of this study was to report the rates of documented oral and oropharyngeal manifestations among these patients and to determine the association of these findings with other MIS-C symptoms.
Methods
The authors conducted a retrospective review of pediatric patients with COVID-19 who were admitted to the Morgan Stanley Children’s Hospital of NewYork-Presbyterian. Patients fulfilling the Centers for Disease Control and Prevention criteria for MIS-C were included in this study. The documented signs, symptoms, and laboratory values were collected and compared with the presence of oral or oropharyngeal findings.
Results
The mean (standard deviation) age of MIS-C patients was 9.0 (5.0) years (range, 1.3-20.0 years), and there was no obvious sex difference (51.1% male, 48.9% female). With respect to oral findings, 23 patients (48.9%) had red or swollen lips, whereas only 5 (10.6%) had a strawberry tongue. Oral or oropharyngeal findings were associated significantly with the presence of systemic rash (P = .04) and conjunctivitis (P = .02).
Conclusions
The presence of oral or oropharyngeal changes may be an early indicator of MIS-C and should be considered suggestive of MIS-C in the setting of COVID-19 infection.
Practical Implications
Dental care providers may play an integral role both in the early detection of oral manifestations of MIS-C and in the identification of oral lesions in hospitalized patients with confirmed MIS-C.
Key Words: COVID-19, MIS-C, multisystem inflammatory syndrome in children, strawberry tongue, Kawasaki disease, pandemic
Abbreviation Key: CDC, Centers for Disease Control and Prevention; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; KD, Kawasaki disease; MIS-C, Multisystem inflammatory syndrome in children; MSCHONY, Morgan Stanley Children’s Hospital of NewYork-Presbyterian; RT-PCR, Reverse transcription polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Coronaviruses are enveloped, nonsegmented, positive sense, RNA viruses belonging to the Coronaviridae family.1, 2, 3 Although most coronaviruses cause mild cold or flulike symptoms, 2 betacoronaviruses (severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus) have resulted in more serious illnesses in 2002 and 2012, respectively.4 , 5 Since December 2019, the novel strain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, has caused a global pandemic.6, 7, 8 Whereas older adults and immunocompromised patients have experienced substantial symptoms from this infection, children and adolescents with COVID-19 generally are asymptomatic and experience only mild respiratory symptoms.9, 10, 11, 12, 13, 14 Despite this observation, an alarming new trend in pediatric COVID-19 infections has been reported. A growing body of literature supports the association between COVID-19 and a postviral immunologic reaction resulting in a multisystem inflammatory syndrome in children (MIS-C).15, 16, 17, 18, 19, 20
The case definition of MIS-C according to the Centers for Disease Control and Prevention (CDC) is a patient younger than 21 years with fever, laboratory evidence of inflammation, and clinical evidence of severe illness necessitating hospitalization, including involvement of 2 or more organ systems.21 These patients also are required to be positive for SARS-CoV-2 infection, confirmed either via reverse transcription polymerase chain reaction (RT-PCR), serology, or antigen testing, and to be without any other plausible cause for their symptoms.21 A fever is defined as a recorded temperature of 38.0°C or higher lasting for at least 24 hours. Laboratory evidence of inflammation includes measurements such as elevated levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and D dimers. Although not all patients with MIS-C are expected to have the same signs and symptoms, clinical features may include fatigue, rash, oropharyngeal erythema, cardiac abnormalities, and dilation of conjunctival blood vessels.22 There have been noted similarities between the defining clinical and laboratory features of MIS-C and Kawasaki disease (KD), although the 2 are considered distinct entities.22 , 23
KD is the most common primary vasculitis in childhood.24, 25, 26 Although the exact etiology of KD is unknown, studies from 2014, 2015, and 2020 suggest that it may be a virally induced illness.27, 28, 29 Diagnosis of KD requires the presence of fever lasting for more than 5 days with at least 4 of the following 5 physical examination findings: conjunctival injection, oral mucous membrane changes (including erythema of the lip vermilion and labial mucosa, erythema of the oral and oropharyngeal mucosa, and strawberry tongue), peripheral extremity changes (erythema, edema), polymorphous rash, or cervical lymphadenopathy.30 Although laboratory results are nonspecific, patients with KD also usually will have elevated CRP, ESR, and D dimers levels.26 KD is considered a diagnosis of exclusion.25 , 31 , 32
MIS-C appears to share many similarities with KD, but it is considered a distinct diagnosis.33 Oral findings are a prominent feature of KD, and, from reports published in 2020, oral mucous membrane changes also appear to be an important finding in MIS-C.16 , 34 One of the more frequently reported oral manifestations of KD is the presence of strawberry tongue.28 , 35 Strawberry tongue describes a hyperplastic appearance of the fungiform papilla set against either a white (white strawberry tongue) or erythematous (red strawberry tongue) background. This manifestation alone is not diagnostic for KD and may be seen in patients with food or medication allergies or in other infectious conditions such as scarlet fever.36 , 37
Unlike KD, in MIS-C the precise incidence of oral findings and their clinical and prognostic significance is unknown. Whereas dentists and other oral health care professionals are accustomed to documenting oral mucosal pathologies, frontline providers may experience difficulty detecting these subtle but potentially important changes. The purpose of our study was to review the incidence and clinical significance of oral and oropharyngeal findings among pediatric patients with MIS-C. Our aims were to report the rates of documented oral and oropharyngeal findings in these patients and to determine the association of these findings with other MIS-C symptoms.
Methods
We conducted a cross-sectional study of all patients with COVID-19 with MIS-C hospitalized at the Morgan Stanley Children’s Hospital of NewYork-Presbyterian (MSCHONY) in New York, New York, from March 15 through June 1, 2020. In this study, we defined MIS-C according to the CDC criteria21; namely, all included patients were 21 years or younger, had a documented fever (≥ 38.0°C) of prolonged duration, had laboratory evidence of inflammation, required hospitalization, had multiorgan involvement, and had a confirmed positive COVID RT-PCR or serology test results. The charts were reviewed for patient demographics such as age and sex, as well as pertinent signs and symptoms, which were obtained from the review of systems and physical examination at the time of admission. We assumed the absence of chart documentation to be a negative response. We conducted summary statistical analyses to determine the overall prevalence of various subjective and objective findings within this patient sample. We conducted comparisons between oral and oropharyngeal findings and all other study variables to reveal any descriptive associations. We conducted univariate comparisons using χ2 and independent sample t tests. We considered a P value below .05 as statistically significant. We conducted this study with the approval of and in compliance with the Columbia University Irving Medical Center’s Institutional Review Board (protocol AAAT0723).
Results
As of June 1, 2020, 150 patients 21 years or younger tested positive for SARS-CoV-2 at MSCHONY. Of these 150 patients, 47 received a diagnosis of MIS-C in the setting of COVID-19 from their infectious disease clinical care team. These 47 patients represented our study cohort. Of the 47 patients who had MIS-C, 100.0% had more than 5 days of fever above 38.0°C. The mean (standard deviation) age at diagnosis was 9.0 (5.0) years (range, 1.3-20.0 years), and both sexes were affected equally (51.1% male, 48.9% female). Twenty-seven patients (57.5%) had documented conjunctivitis, 32 (68.1%) had a systemic rash, 6 (12.8%) had extremity edema, and 9 (19.2%) had cervical lymphadenopathy. Twenty-three patients (48.9%) had red or swollen lips, but only 5 (10.6%) had documented strawberry tongue. Six (12.8%) patients had cranial nerve palsy. Many patients also experienced nonspecific constitutional symptoms such as diarrhea (18 patients, 38.3%), vomiting (24 patients, 51.1%), cough (7 patients, 14.9%), irritability (7 patients, 14.9%), or rhinorrhea (3 patients, 6.4%). Most patients had elevated CRP (44 patients, 93.6%), ESR (35 patients, 87.5%), and D dimers (43 patients, 93.5%) levels as would be expected with a systemic inflammatory condition (Table 1 ). Overall, oral or oropharyngeal findings were identified in more than one-half (55.3%) of patients with MIS-C. These findings were associated significantly with the presence of a systemic rash (P = .04), conjunctivitis (P = .02), and absence of a cough (P = .02) (Table 2 ). The presence of oral or oropharyngeal changes was not associated with coexisting cardiac conditions such as myocarditis (P = .33) or pericardial effusions (P = .55).
Table 1.
Summary of clinical and laboratory findings in patients with multisystem inflammatory syndrome in children.∗
| FINDINGS | CASES, NO. (%) |
|---|---|
| Age, y (Standard Deviation) | 9.0 (5.0) |
| Male, No. (%) | 24 (51.1) |
| Review of Systems | |
| Diarrhea | 18 (38.3) |
| Vomiting | 24 (51.1) |
| Cough | 7 (14.9) |
| Irritability | 7 (14.9) |
| Rhinorrhea | 3 (6.4) |
| Clinical Examination and Imaging | |
| Fever > 5 days | 47 (100.0) |
| Systemic rash | 32 (68.1) |
| Conjunctivitis | 27 (57.5) |
| Cranial nerve palsy | 6 (12.8) |
| Red, cracked lips | 23 (48.9) |
| Strawberry tongue | 5 (10.6) |
| Other oral manifestations | 7 (14.9) |
| Myocarditis | 17 (36.2) |
| Pericardial effusion | 6 (12.8) |
| Extremity edema | 6 (12.8) |
| Arthritis | 4 (8.5) |
| Cervical lymphadenopathy | 9 (19.2) |
| Laboratory Tests | |
| COVID reverse transcription polymerase chain reaction + | 22 (46.8%) |
| COVID serology | 39 (83.0%) |
| Elevated C-reactive protein | 44 (93.6%) |
| Elevated erythrocyte sedimentation rate | 35 (87.5%) |
| Elevated D dimer | 43 (93.5%) |
N = 47.
Table 2.
Review of oral and oropharyngeal manifestations in patients with multisystem inflammatory syndrome in children.
| FINDINGS | ORAL OR OROPHARYNGEAL FINDINGS, NO. (%) (N = 26) | NO ORAL OR OROPHARYNGEAL FINDINGS, NO. (%) (N = 21) | P VALUE |
|---|---|---|---|
| Mean Age, y (95% Confidence Interval) | 7.8 (5.7 to 9.8) | 10.5 (8.5 to 12.5) | .06 |
| Male, No. (%) | 11 (42.3) | 13 (61.9) | .18 |
| Review of Systems | |||
| Diarrhea | 8 (30.8) | 10 (47.6) | .24 |
| Vomiting | 13 (50.0) | 11 (52.4) | .87 |
| Cough | 1 (3.9) | 6 (28.6) | .02∗ |
| Irritability | 5 (19.2) | 2 (9.5) | .35 |
| Rhinorrhea | 2 (7.7) | 1 (4.8) | .68 |
| Clinical Examination and Imaging | |||
| Fever > 5 days | 26 (100.0) | 21 (100.0) | Not applicable |
| Systemic rash | 21 (80.8) | 11 (52.4) | .04∗ |
| Conjunctivitis | 19 (73.1) | 8 (38.1) | .02∗ |
| Cranial nerve palsy | 4 (15.4) | 2 (9.5) | .55 |
| Myocarditis | 11 (42.3) | 6 (28.6) | .33 |
| Pericardial effusion | 4 (15.4) | 2 (9.5) | .55 |
| Extremity edema | 5 (19.2) | 1 (4.8) | .14 |
| Arthritis | 3 (11.5) | 1 (4.8) | .41 |
| Cervical lymphadenopathy | 7 (26.9) | 2 (9.5) | .13 |
| Laboratory Tests | |||
| Elevated C-reactive protein | 25 (96.2) | 19 (90.5) | .43 |
| Elevated erythrocyte sedimentation rate | 19 (86.4) | 16 (88.9) | .81 |
| Elevated D dimer | 22 (88.0) | 21 (100.0) | .10 |
P < .05
Discussion
The association between MIS-C and COVID-19 was first described anecdotally but has been reported more extensively in the global literature.17, 18, 19 , 38 Toubiana and colleagues20 documented 21 children (median age, 7.9 years) in Paris, France, with MIS-C. These patients were treated with immunoglobulin and corticosteroid therapy and had favorable outcomes.20 Verdoni and colleagues39 reported a 30-fold increase in the incidence of Kawasaki-like disease in the Bergamo province of Italy in early May.39 Although that report did not refer specifically to MIS-C, the patient population analyzed all had positive COVID RT-PCR or serology test results and, therefore, would more appropriately be designated as MIS-C according to the CDC classification. In the United States, 6 pediatric-aged patients with MIS-C were initially identified in Philadelphia, Pennsylvania.19 On May 12, New York state health officials announced a report of approximately 100 cases of MIS-C.40 The high rate of MIS-C documented in our study cohort (47 patients) may reflect the selection bias of our particular institution; the pediatric population being admitted to MSCHONY during this time was composed of symptomatic patients with COVID-19 at the height of the pandemic in New York City.
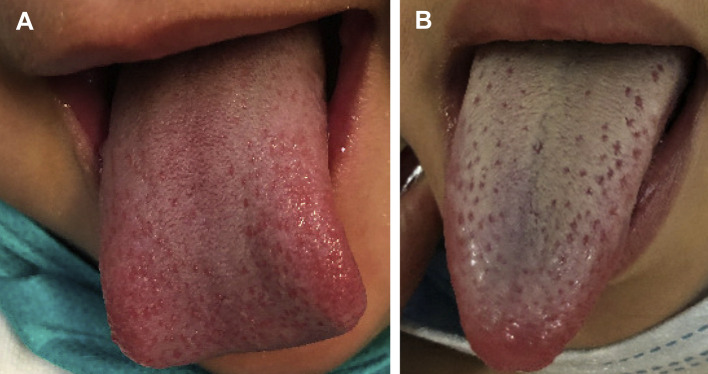
The purpose of our study was to report the incidence of oral and oropharyngeal findings in MIS-C and to determine the clinical significance of these changes. In our cohort of 47 patients, 23 had documented swelling, redness, or cracking of the labial mucosa on examination. Only 5 had strawberry tongue. Although it is unclear whether this represents a true negative finding or an error of omission, the lack of documented strawberry tongue in our patient cohort was noted substantially more frequently than other extraoral diagnostic criteria for MIS-C, such as fever (47 patients), systemic rash (32 patients), and conjunctivitis (27 patients). In 2 of the 5 cases of confirmed strawberry tongue, intraoral photographs were obtained. The findings are more subtle when the hyperplastic papilla appear against an erythematous background (red strawberry tongue) (Figure , A) as opposed to when they manifest in a patient with white strawberry tongue (Figure, B). A cursory examination in a small child could easily result in these changes going undetected. Furthermore, the discordance between documented labial findings (48.9%) and strawberry tongue (10.6%) may suggest that the health care providers who are performing these evaluations experience difficulty detecting intraoral lesions. If this is the case, it reinforces the value of a dental care provider in the workup and management of MIS-C. None of the patients in our sample were assessed by a dentist or dental specialist during their inpatient course at MSCHONY for evaluation of oral manifestations of MIS-C.
Figure.
Clinical photographs of red strawberry tongue appearing as hyperplastic papilla against an erythematous dorsal tongue (A) and white strawberry tongue (B) manifesting as hyperplastic papilla against a white coating of the dorsal tongue.
In addition to labial mucosal alterations and strawberry tongue, 7 patients in our cohort also had other oral changes noted on clinical examination, which we categorized as “other oral manifestations.” Three patients had reported blisters or sores in their mouths; however, neither the site, size, nor clinical appearance was recorded in any of the 3 cases. The lack of additional information makes it difficult to assess whether these lesions were viral or traumatic in etiology. One patient had a lesion on the inner lip, and 1 had reported symptoms of mouth pain. Specific mention of posterior oropharyngeal erythema was made for 1 patient. There was 1 documented case of smile asymmetry and tongue deviation. This was presumed to be caused by cranial nerve palsy, which is another potential characteristic of MIS-C. Cranial nerve palsy was documented in 6 patients in our study, and oral or oropharyngeal findings were reported in 4 of them.
The documented mean age at diagnosis of 9 years in our patient cohort is consistent with other studies reporting a higher average age of diagnosis in patients with MIS-C than in those with KD, which is traditionally diagnosed in children younger than 5 years with a peak incidence at approximately 10 months.16 , 22 The relationship between MIS-C and KD is unclear, and there is undoubtedly overlap between the 2 with regard to clinical and laboratory findings. To this end, some have questioned whether MIS-C and KD may in fact be the same entity, with some cases of MIS-C being categorized as either incomplete or complete manifestations of KD.41, 42, 43, 44 Although it is beyond the scope of our article to speculate on the uniqueness of these 2 entities, oral manifestations more commonly were associated with the other diagnostic criteria for both MIS-C and KD, including systemic rash (P = .04) and conjunctivitis (P = .02). Strawberry tongue, which is a characteristic albeit nonspecific oral manifestation of KD, was only reported in 5 of the 47 patients with MIS-C. As previously stated, it is unclear whether this means that strawberry tongue is not strongly associated with MIS-C or if the lack of documentation suggests challenges with the diagnosis of this sometimes subtle oral mucosal manifestation. Also of statistical significance was the lack of oral findings in patients with a documented cough (P = .02). Only 7 patients (14.9%) in our cohort reported cough in association with MIS-C, further suggesting that the documented oral manifestations in our cohort may represent a reproducible feature of the clinical manifestation of MIS-C.
Dental care providers potentially can play an important role in the initial diagnosis of MIS-C, both in the inpatient and outpatient settings. As dental practices begin to reopen across the country and elective treatments resumes, it is possible that dentists will encounter patients who are or have received a diagnosis of COVID-19 with features of MIS-C.45 This is especially true for those working in pediatric or family dental practices. Given that oral manifestations are believed to be some of the first signs of MIS-C,45 dental care professionals may be consulted in the inpatient setting to evaluate subtle changes of the oral mucosa. Equally important, dental care providers also can help frontline workers rule out changes unrelated to MIS-C. Strawberry tongue is 1 such example of an oral manifestation of MIS-C that may be difficult to detect and can be confused with other similar-appearing lesions of the dorsal tongue, such as oral thrush or white-coated tongue. Early diagnosis and close monitoring of MIS-C are important because, similar to KD, cardiac changes have been noted.18 , 46 In the study by Toubiana and colleagues,20 moderate coronary artery dilation was detected in 24% of patients with MIS-C.20 Evidence reported in 2020 by Belhadjer and colleagues18 suggests myocardial involvement with acute heart failure is likely due to myocardial stunning or edema rather than inflammatory damage.18 Among our 47 patients, 17 (36.2%) had myocarditis and 7 (12.8%) had pericardial effusion; however, neither of these cardiac changes were associated significantly with oral or oropharyngeal changes (Table 2). Although the long-term sequelae of MIS-C are still unclear, accurate and early detection of patients with MIS-C may help prevent future complications.
Conclusions
The incidence of MIS-C is likely to increase as the COVID-19 pandemic continues to evolve. Oral changes among patients with diagnosed MIS-C were seen with relative frequency (approximately one-half of our patient cohort) and were positively associated with other extraoral diagnostic criteria for MIS-C, including fever and conjunctivitis. Therefore, the accurate determination of oral changes in patients with MIS-C may have important diagnostic implications. Given the prevalence and apparent clinical significance of oral and oropharyngeal changes, dentistry can play an integral role both in the early detection of oral manifestations of MIS-C and in the identification of oral lesions in hospitalized patients with confirmed MIS-C. Dental care providers should be cognizant of the potential oral manifestations of MIS-C and, in the appropriate clinical setting, be willing to evaluate high-risk patients.
Biographies
Dr. Halepas is a resident, Department of Oral and Maxillofacial Surgery, Columbia University Irving Medical Center, New York City, New York.
Dr. Lee is a resident, Department of Oral and Maxillofacial Surgery, Columbia University Irving Medical Center, New York City, New York.
Dr. Myers is an assistant professor, Department of Pediatric Dentistry, Columbia University Irving Medical Center, New York City, New York.
Dr. Yoon is an assistant professor, Department of Pediatric Dentistry, Columbia University Irving Medical Center, New York City, New York.
Dr. Chung is a Kennedy Family Professor of Pediatrics and Medicine, Columbia University Irving Medical Center, New York City, New York.
Dr. Peters is an assistant professor, Division of Oral and Maxillofacial Pathology, Columbia University Irving Medical Center, New York City, New York.
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Footnotes
Disclosures. None of the authors reported any disclosures.
References
- 1.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo P.C.Y., Lau S.K.P., Huang Y., Yuen K.-Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. 2009;234(10):1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 3.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:1024–1033. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohrabi C., Alsafi Z., O’Neill N. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X., Zhang L., Du H. et al; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castagnoli R., Votto M., Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 11.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. 2020;92(7):747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhochak N., Singhal T., Kabra S.K., Lodha R. Pathophysiology of COVID-19: why children fare better than adults? Indian J Pediatr. 2020;87(7):537–546. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen K.L., Yang Y.H., Jiang R.M. et al; China National Clinical Research Center for Respiratory Diseases; National Center for Children’s Health, Beijing, China; Group of Respirology, Chinese Pediatric Society, Chinese Medical Association; Chinese Medical Doctor Association Committee on Respirology Pediatrics; China Medicine Education Association Committee on Pediatrics; Chinese Research Hospital Association Committee on Pediatrics; China Non-government Medical Institutions Association Committee on Pediatrics; China Association of Traditional Chinese Medicine, Committee on Children’s Health and Medicine Research; China News of Drug Information Association, Committee on Children’s Safety Medication; Global Pediatric Pulmonology Alliance. Updated diagnosis, treatment and prevention of COVID-19 in children: experts’ consensus statement (condensed version of the second edition) World J Pediatr. 2020;16(3):232–239. doi: 10.1007/s12519-020-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein L.R., Rose E.B., Horwitz S.M. et al; for the Overcoming COVID-19 Investigators, and the CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrero-Hernández M., García-Salido A., Leoz-Gordillo I. Severe SARS-CoV-2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J. 2020;39(8):e195–e198. doi: 10.1097/INF.0000000000002777. [DOI] [PubMed] [Google Scholar]
- 18.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 19.Chiotos K., Bassiri H., Behrens E.M. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9(3):393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) cdc.gov/mis-c/hcp/ Available at: Accessed June 14, 2020.
- 22.Rowley A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder A.R., Wilson K.M., Ralston S.L. COVID-19 and Kawasaki disease: finding the signal in the noise. Hosp Pediatr. 2020;10(10):e1–e3. doi: 10.1542/hpeds.2020-000356. [DOI] [PubMed] [Google Scholar]
- 24.Schnabel A., Hedrich C.M. Childhood vasculitis. Front Pediatr. 2018;6:421. doi: 10.3389/fped.2018.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shackelford P.G., Strauss A.W. Kawasaki syndrome. N Engl J Med. 1991;324(23):1664–1666. doi: 10.1056/NEJM199106063242311. [DOI] [PubMed] [Google Scholar]
- 26.Sundel R. Kawasaki disease: clinical features and diagnosis. https://www.uptodate.com/contents/kawasaki-disease-clinical-features-and-diagnosis Available at: Accessed June 14, 2020.
- 27.Rowley A.H., Baker S.C., Arrollo D. A protein epitope targeted by the antibody response to Kawasaki disease. J Infect Dis. 2020;222(1):158–168. doi: 10.1093/infdis/jiaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang L.Y., Lu C.Y., Shao P.L. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113(3):148–154. doi: 10.1016/j.jfma.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnier J.L., Anderson M.S., Heizer H.R., Jone P-N, Glodé M.P., Dominguez S.R. Concurrent respiratory viruses and Kawasaki disease. Pediatrics. 2015;136(3):e609–e614. doi: 10.1542/peds.2015-0950. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [in Japanese] Arerugi. 1967;16(3):178–222. [PubMed] [Google Scholar]
- 31.Singh S., Jindal A.K., Pilania R.K. Diagnosis of Kawasaki disease. Int J Rheum Dis. 2018;21(1):36–44. doi: 10.1111/1756-185X.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrindle B.W., Rowley A.H., Newburger J.W. et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 33.Cheung E.W., Zachariah P., Gorelik M. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dufort E.M., Koumans E.H., Chow E.J. et al; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saguil A., Fargo M., Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. 2015;91(6):365–371. [PubMed] [Google Scholar]
- 36.Adya K.A., Inamadar A.C., Palit A. The strawberry tongue: what, how and where? Indian J Dermatol Venereol Leprol. 2018;84(4):500–505. doi: 10.4103/ijdvl.IJDVL_57_17. [DOI] [PubMed] [Google Scholar]
- 37.Ryu S., Chun B.C. Investigation of scarlet fever outbreak in a kindergarten. Infect Chemother. 2018;50(1):38–42. doi: 10.3947/ic.2018.50.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belluck P. A new coronavirus threat to children. New York Times. May 12, 2020 18. [Google Scholar]
- 41.Panupattanapong S, Brooks EB. New spectrum of COVID-19 manifestations in children: Kawasaki-like syndrome and hyperinflammatory response [published online ahead of print June 3, 2020]. Cleve Clin J Med. 10.3949/ccjm.87a.ccc039. [DOI] [PubMed]
- 42.Ebina-Shibuya R., Namkoong H., Shibuya Y., Horita N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial Kawasaki disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harahsheh A.S., Dahdah N., Newburger J.W. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones V.G., Mills M., Suarez D. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 45.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99(5):481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capone C.A., Subramony A., Sweberg T. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]