Abstract
Half a year after its emergence, severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) has resulted in a pandemic, with cases continuing to increase in nearly every country. Surges in coronavirus disease of 2019 (COVID-19) cases have clearly had profound effects on current cancer treatment paradigms. Considering the effect of antineoplastic treatment and the immunosuppressive properties of cancer itself, cancer patients are deemed to be more vulnerable to SARS-CoV-2. Hence, the specific risk of SARS-CoV-2 must be carefully weighed against the benefit of antineoplastic treatment for cancer patients in the COVID-19 era. In this review, we discuss the current evidence in this important field, and in particular, the effect of SARS-CoV-2 on antineoplastic treatment.
Keywords: COVID-19, Cancer, Mortality, Antineoplastic treatment
1. Introduction
Severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) is a novel beta-coronavirus and the causative agent of coronavirus disease 2019 (COVID-19) [1,2]. As of 26 July 2020, over 16 million cases of COVID-19 have been confirmed globally, with nearly 650,000 deaths [3]. Recently, several studies revealed a recurrent pattern of D614 G mutation of SARS-CoV-2 at multiple geographic levels, which enhances viral transmission ability [[4], [5], [6]]. This implies that the virus will probably persist for the long term.
Approximately 7%–8% of hospitalized COVID-19 infected patients also had a malignant disease [[7], [8], [9], [10], [11], [12]]. Additionally, these included cancer patients with advanced age, coexistent comorbidities, and immunocompromised conditions, which are all established factors associated with COVID-19 risk [8,13,14]. Hence, in the past few months of the COVID-19 era, treatment for cancer patients has received increasing attention from oncologists [15,16]. Studies on cancer patients with COVID-19 have already been published. A literature search was performed in PubMed using the following descriptors “COVID-19”, “SARS-CoV-2”, “Cancer” and “Tumor”. Based on these data, we have drafted some clinical characteristics of cancer patients with COVID-19 and reassessed the specific risk of SARS-CoV-2 in cancer treatment.
2. Clinical observations
2.1. Clinical outcomes
In February 2020, an early report from Liang et al. [17]. reported a cancer prevalence of 1% (95% confidence interval [CI], 0.61% to 1.65%) among 1,590 patients with COVID-19, and these patients had a higher risk of severe events compared with those without cancer (39% vs. 8%, p = 0.0026). It is currently believed that patients with cancer show a more critical COVID-19 situation than individuals without cancer. This may result from the following factors: (1) the majority of cancer patients present with advanced age and coexistent comorbidities, which have been confirmed as high-risk factors [8,10,13,18]; (2) the immunosuppressive properties of cancer itself; and (3) treatment options, such as chemotherapy and steroid therapy, could induce systemic immunosuppressive states. Therefore, an increasing number of research teams from different regions are joining the effort to explore the specific relationships between COVID-19 and cancer more deeply (Table 1 ).
Table 1.
Recent studies investigating the clinical outcomes and risk factors of COVID-19 patients with cancer.
| Ref. | Region | Study design | Time period | Sample size | Mortality | Primary endpoint | Cancer stage | Cancer type | Other findings |
|---|---|---|---|---|---|---|---|---|---|
| Dai et al. [22] | Hubei, China | Retrospective | 1 Jan 2020–24 Feb 2020 | 105 | 11.4% | Severe outcomes# | Metastatic cancer/stage IV (HR = 2·48, p = 0·01) |
- Hematologic cancer (HR = 6·30, p < 0.01) - Lung cancer (HR = 2.59, p < 0.01) |
Lung metastases (HR = 2·58, p < 0.01) |
| Zhang et al. [28] | Hubei, China | Retrospective | 13 Jan 2020–26 Feb 2020 | 28 | 28.6% | Severe outcomes# | Metastatic cancer/stage IV (HR = 0.509, p = 0.194) |
― | Patchy consolidation (HR = 5·438, p = 0.01) |
| Tian et al. [21] | Hubei, China | Retrospective | 13 Jan 2020–18 Mar 2020 | 232 | 20% | Severe outcomes# | Stage IV (multivariable OR = 2.60, p = 0.039) |
― | Time since diagnosis (multivariable HR = 0.029, p<0.001) |
| Yang et al. [23] | Hubei, China | Retrospective | 13 Jan 2020–24 Mar 2020 | 205 | 19.5% | Mortality | Stage III/IV (univariable OR = 3.38, p = 0.011) |
Hematologic cancer (HR = 3.28, p = 0.0009) | Male sex (multivariable OR = 3.86, p = 0.0033) |
| Kuderer and Rivera et al (CCC19) [19,27] | USA, Canada, and Spain | Prospective | 17 March 2020–26 June 2020 | 2186 | 15% | Mortality | Active cancer/progressing (multivariable OR = 5.20) | No specific cancer type was associated with mortality | ― |
| Mehta et al. [24] | New York, USA | Retrospective | 18 March 2020–8 April 2020 | 218 | 28% | Mortality | Metastatic stage (p = 0.06) | - Lung cancer (mortality rate: 55%) - Hematologic cancer (mortality rate: 25%) |
― |
| Lee et al. (UKCCMP) [25,26] | UK | Prospective | 18 March 2020–8 May 2020 | 1044 | 30.6% | Mortality | Metastatic stage (multivariable OR = 1.34, p = 0.579) | Hematologic cancer (OR = 1.74, p < 0.01) | Age (p < 0.0001) |
| Garassino et al. (TERAVOLT) [29,30] | International multicenter | Retrospective | 26 March 2020–11 Sep 2020 | 326 (thoracic cancer) | 32% | Mortality | Metastatic cancer/stage IV (OR = 1.9, p < 0.001) | ― | Smoking history (multivariable OR = 1.8) |
| Pinato et al. (OnCOVID) [20] | European | Retrospective | 26 February 2020–7 May 2020 | 890 | 33.6% | Mortality | Active cancer (multivariable HR = 1.81, p < 0.0001) | - Genitourinary cancer (mean OS: 22 days) - Hematologic cancer (mean OS: 24 days) |
― |
Abbreviations: COVID-19, coronavirus disease 2019; HR, hazard ratio; OR, odds ratio; OS, overall survival.
#Severe outcomes including admission to an ICU, development of severe or critical symptoms, the use of mechanical ventilation, or death.
Currently, the global COVID-19 mortality rate is about 4·1%, while the data show four-fold to five-fold differences between the countries with the highest and lowest rates [3]. Even so, COVID-19 patients with a cancer history showed significantly worse prognosis than those without cancer. The short-term mortality for COVID-19 patients with cancer was approximately 11% to 28% (Table 1). Moreover, it seems that the mortality rate did not qualitatively change with different enrolment time periods and geographical distribution.
Other prognostic factors, including cancer state and cancer type, are worthy of concern. It is easy to understand that metastatic stage or active cancer patients have a significantly worse prognosis than early stage or remission cancer patients. Indeed, according to results from the COVID-19 and Cancer Consortium registry database, active cancer (progressing vs remission) was an independent factor associated with increased 30-day mortality (odds ratio = 5.20, 95% CI: 2.77–9.77) [19]. Similar results were observed in the COVID study [20]. As previously mentioned, a multi-center retrospective study showed that, in COVID-19 cases, confirmed time since cancer diagnosis (1–5 years vs. < 1 year) was significantly associated with non-severe disease outcome (hazard ratio = 0·227, 95% CI: 0.116–0.446, p < 0.0001) [21]. This suggests that the more active the tumor, the more serious the COVID-19 condition. In addition, there is controversy regarding whether specific cancer type is associated with the COVID-19 clinical outcome. As summarized in Table 1, several retrospective studies pointed out that hematologic cancer and lung cancer were associated with worse prognosis than other cancer types [[22], [23], [24], [25], [26]]. However, results from the CCC19 cohort demonstrated a negative association between cancer type and the COVID-19 clinical outcome [19,27].
Thus far, these studies clearly indicate that COVID-19 patients with cancer had a 2.5- to 7-fold increase in risk of death and severe outcomes compared to those without cancer. Particular attention should be given to patients with metastatic stage or active cancer.
2.2. Antineoplastic treatment and COVID-19
Whether recent antineoplastic treatment would aggravate the COVID-19 condition is another important question. The qualitative results of the prognostic value of recent antineoplastic treatment for COVID-19 patients are summarized in Table 2 . Interestingly, contradictory results have been observed.
Table 2.
Recent studies investigating the effect of recent antineoplastic treatment for COVID-19 patients with cancer.
| Ref | Sample size | Outline of enrolled cancer patients | The prognostic role of antineoplastic treatment |
|---|---|---|---|
| Dai et al. [22] | 105 | - Stage IV: 21.0% - 36 patients (34.3%) received antineoplastic treatment (≤40 days) # |
- Immunotherapy: higher rate of death 33.3% - Surgery: higher rate of death 25.0% |
| Zhang et al. [28] | 28 | - Stage IV: 35.7% - 6 patients (21.4%) received antineoplastic treatment (≤14 days)# |
- If the last antineoplastic treatment was within 14 days, it significantly increased the risk of developing severe events (HR = 4.079, p = 0.037) |
| Tian et al. [21] | 232 | - Stage IV: 15% | - Targeted therapy or immunotherapy (multivariable OR = 3.29, p = 0.015) - Time interval since last chemotherapy to hospital admission (multivariable HR = 3.51, p = 0.046) |
| Yang et al. [23] | 205 | - Stage III/IV: 27% - 54 patients (26.3%) received antineoplastic treatment (≤28 days) |
- Chemotherapy (multivariable OR = 3.51, p = 0.026) |
| Kuderer and Rivera et al (CCC19) [19,27] | 2186 | - Present, progressive disease: 11% - 371 patients (40%) received antineoplastic treatment (≤28 days) |
- Type of antineoplastic therapy and recent surgery were not associated with mortality |
| Mehta et al. [24] | 218 | - Stage IV/metastatic: 19.3% - 47.1% patients received antineoplastic treatment (≤30 days) |
- Chemotherapy, radiotherapy, and immunotherapy were not associated with mortality |
| Lee et al. (UKCCMP) [25,26] | 1044 | - Stage IV/metastatic: 43% - 66% patients received antineoplastic treatment (≤28 days) |
- Recent chemotherapy (≤4 weeks) for patients with hematologic malignancies (OR = 2.09, p = 0.028) |
| Garassino and Trama et al. (TERAVOLT) [29,30] |
326 (thoracic cancer) | - Non-small cell lung cancer: 76% - 74% patients received antineoplastic at the time of COVID-19 diagnosis, and 37% received immune checkpoint inhibitors |
- Type of antineoplastic therapy were not associated with mortality |
| Pinato et al. (OnCOVID) [20] | 890 | - Advanced stage: 39.4% - Active cancer: 62.5% - 53.8% patients were on antineoplastic therapy at the time of COVID-19 diagnosis |
- Provision of chemotherapy, targeted therapy, and immunotherapy did not worsen mortality |
COVID-19 = coronavirus disease, 2019. HR = hazard ratio. OR = odd ratio.
Some possible explanations for the apparent discrepancies are as follows: first, the observed heterogeneity between studies, especially the proportion of stage IV patients and patients receiving antineoplastic treatment. It is interesting to note that studies with negative findings had a higher proportion of patients who recently received antineoplastic treatment. Second, positive findings were obtained from retrospective cohort studies with an early enrolled time period in China. All enrolled patients were from the Hubei province, the early stage epicenter of the pandemic in China. Hence, selection bias in these retrospective studies should not be ignored. Moreover, based on phylogenetic network analysis, the prevalence and genotype distribution of SARS-CoV-2 in East Asians, Europeans, and Americans varies [31,32]. Different genomic clusters of SARS-CoV-2 may cause different prognostic effects that remain unknown.
Two retrospective studies from China found a significant correlation between time interval since last antineoplastic treatment and COVID-19 prognosis [21,28]. This suggests that shorter time intervals are associated with a greater effect on COVID-19 outcomes. This is consistent with findings that COVID-19 patients with active cancer status had a worse prognosis.
Overall, these studies revealed that no conclusion can be drawn as to whether recent antineoplastic treatment aggravates COVID-19 disease. However, there are reasons for caution with patients who received antineoplastic treatment within the previous two weeks, especially those with significant treatment-related side effects.
2.3. Immunotherapy and COVID-19
Given their profound immunomodulatory activity, concerns about immunotherapy interference with the clinical course of SARS-CoV-2 infection have been raised in the early phase of the outbreak [33,34]. Lovly et al. [35] reported a case of an extensive small cell lung cancer patient treated with carboplatin, etoposide, and atezolizumab. The patient developed rapidly fatal pneumonitis within three days of the first treatment dose. Unfortunately, no therapeutic benefits were observed in this patient after high-dose steroids and infliximab were administered out of concern for immune-related pneumonitis. The patient was later diagnosed with SARS-CoV-2 infection by nasal swab reverse transcription-polymerase chain reaction; the immunoglobulin M seropositivity for SARS-CoV-2 suggested that the viral infection occurred before the patient received antineoplastic treatment. Although the reason for the fatal pneumonitis is not known, the interaction between SARS-CoV-2 infection and immune checkpoint inhibitors should be considered.
In a recent retrospective study from the Memorial Sloan Kettering Cancer Center, Luo et al. [36] investigated 69 COVID-19 patients with lung cancer, where 59% had previously received programmed cell death protein 1 blockade therapy (median last dose to COVID-19 diagnosis 45 days, range 4–820 days). Moreover, 200 patients with COVID-19 and thoracic cancers were enrolled in the thoracic cancers international COVID-19 collaboration registry, an international cohort study. Seventy-four percent were on antineoplastic therapy at the time of COVID-19 diagnosis, 37% of which included immune checkpoint inhibitors. The multivariate analysis showed that any type of antineoplastic therapy, including immune checkpoint inhibitors, was associated with an increased risk of death. These results were consistent with the majority of studies shown in Table 2 and suggest that there was no significant difference in severity regardless of PD-1 blockade exposure.
3. Adjustments of cancer treatment paradigm in the COVID-19 era
3.1. Ethical prioritization strategy
Surges in COVID-19 cases are deemed to cause a shortage of medical resources. Not surprisingly, there was a significant decline in the number of cancer-related hospitalizations and operations during the pandemic [[37], [38], [39]]. Based on an analysis of the UK’s National Endoscopy Database, the activity of endoscopy procedures in the COVID-19-impacted period decreased to 12% of pre-COVID-19 levels, and the number of cancers detected weekly decreased by 58% [40]. Moreover, according to a nationwide online survey of cancer patients in the Netherlands, treatment was postponed in 39 of 250 patients (16%) and in 279 of 2391 patients (12%) who were awaiting treatment [41]. Hence, the primary challenge of a cancer treatment paradigm in the COVID-19 era is to ensure that patients receive timely cancer diagnosis and treatment.
As the epidemic progresses, there is a growing body of guidelines, measures, and consensus that provide more or less formal instructions for adjusted cancer care in the context of the COVID-19 pandemic [[42], [43], [44], [45], [46], [47], [48], [49]]. A key issue in these guidelines concerns the ethical problems arising from scarce medical resources in a pandemic [50,51]. Hence, when assessing these guidelines, the crucial issue lies in determining the priority system of different diseases within the ethical framework. In April, the American Society of Clinical Oncology (ASCO) drafted several recommendations for the oncology community to observe during the COVID-19 pandemic, which highlighted that the allocation of scarce resources should be based on maximizing health benefits [52]. The ASCO recommended that a fair and consistent allocation policy should be developed based on acknowledged ethical frameworks [53,54]. Therefore, ethical and rational prioritization strategies were the most important aspects of these instructions, especially for cancer patients who require surgery. Reduced surgical and perioperative care capacities often lead to a delay in the time to surgery [55]. Therefore, different specialties have published several surgical recommendations or guidelines, such as head and neck surgery, thoracic surgery, and colorectal surgery [42,[56], [57], [58], [59], [60], [61], [62]]. Detailed prioritization strategies are embodied in these guidelines. Recently, to better overcome the clinical dilemmas in the COVID-19 era, a global consortium led by the European Society for Medical Oncology published 28 statements on the clinical and technical areas of uncertainty ranging from diagnosis to therapeutic planning [48]. Overall, the ethical approach to cancer patients in the COVID-19 era would follow these clinical practice guidelines and consensus after considering local disease prevalence and medical resources.
3.2. Some points for future cancer treatment paradigms
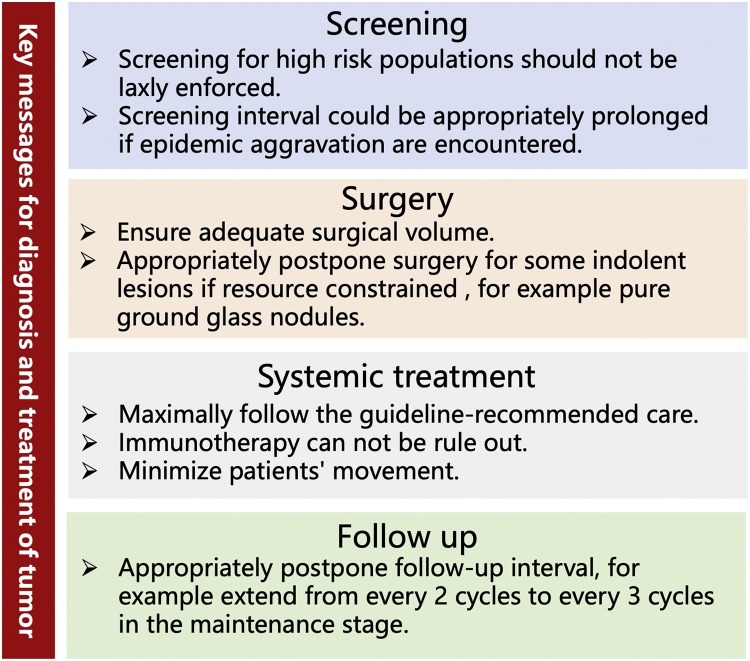
Taken together the results of previous studies provide several take home messages from the clinical perspective (Fig. 1 ). Besides, some key points are suggested to mitigate the massive challenges to future treatment paradigms caused by the pandemic. First, accelerate telehealth services development to ensure the quality of cancer care while avoiding face-to-face contact [[63], [64], [65]]. This is an essential strategy for mitigating the spread of SARS-CoV-2 [66]. In fact, a substantial proportion of hospitals have either partial or full implementation of computerized telehealth capabilities; the major barrier remains as to how to use it. Currently, medical telehealth service in China was limited to treatment decision making among doctors. We should expand the application scenario of telehealth, such as tele-consent, psychological guidance and non-treatment visits. Second, allow oncologists to return to cancer clinical trials and cancer research [[67], [68], [69]]. The pandemic has caused widespread disruption of cancer clinical trials, which could have a profound impact on the survival of cancer patients [70,71]. Prolonged clinical trial closure does not appear to be warranted. Third, early detection of cancer is crucial for secondary cancer prevention [[72], [73], [74]]. Screening for high-risk populations must be firmly implemented in accordance with the guidelines.
Fig. 1.
Several take home messages for diagnosis and treatment of tumor in the COVID-19 era.
4. Conclusions
The COVID-19 pandemic has already had a profound effect on cancer treatment paradigms. As a vulnerable population, cancer patients require more caution in the COVID-19 era. Although many aspects of SARS-CoV-2 and cancer remain unclear, there is no conclusive evidence indicating that antineoplastic treatment aggravates COVID-19 disease. Hence, from a clinical viewpoint, when any kind of treatment represents the optimal choice, it seems unreasonable to deny it to cancer patients or to interrupt its administration due to fears of COVID-19 infection. Under this premise, we cannot stop providing the best available treatment for cancer patients. According to incomplete statistics, there are currently over 200 kinds of COVID-19 vaccine being developed [75], and ten of them has progressed to phase III clinical trials [76]. We hope that the mass vaccination programs could control and end this pandemic.
5. Authors contribution
JTZ, WZZ, and YLW designed the concept and outline of the original draft. JTZ wrote the original manuscript, WZZ and YLW reviewed and supported the editing of the original manuscript. YLW edited the final manuscript.
Funding
This work was supported by the Special Fund of Public Interest by National Health and Family Control Committee (201402031 to Y.L. Wu), Key Lab System Project of Guangdong Science and Technology Department, and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (2012A061400006, 2017B030314120 to Y.L. WU), and Health Collaborative Innovation Major Project from Guangzhou Science and Technology Bureau (201400000001–2 to Y.L. WU). The funding source had no role in the preparation of this manuscript.
Declaration of Competing Interest
The authors have nothing to disclose.
References
- 1.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.
- 4.Korber W.M.F., Gnanakaran S., Yoon H., et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Jackson C.B., Mou H., et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. 2020 doi: 10.1101/2020.06.12.148726. [DOI] [Google Scholar]
- 6.Mansbach R.A., Chakraborty S., Nguyen K., Montefiori D., Korber B., Gnanakaran S. The SARS-CoV-2 spike variant D614G favors an open conformational state. bioRxiv. 2020 doi: 10.1101/2020.07.26.219741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A., et al. Base line characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 13.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argenziano M.G., Bruce S.L., Slater C.L., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Haar J., Hoes L.R., Coles C.E., et al. Caring for patients with cancer in the COVID-19 era. Nat. Med. 2020;26:665–671. doi: 10.1038/s41591-020-0874-8. [DOI] [PubMed] [Google Scholar]
- 16.Ueda M., Martins R., Hendrie P.C., et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J. Natl. Compr. Canc. Netw. 2020;18:1–4. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 17.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinato D.J., Zambelli A., Aguilar-Company, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian J., Yuan X., Xiao J., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K., Sheng Y., Huang C., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Lyw, Cazier Jb, Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/s1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera D.R., Peters S., Panagiotou O.A., et al. Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov. 2020;10:1514–1527. doi: 10.1158/2159-8290.cd-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trama A., Proto C., Whisenant J.G., et al. Supporting Clinical Decision-Making during the SARS-CoV-2 Pandemic through a Global Research Commitment: The TERAVOLT Experience. Cancer Cell. 2020;38:602–604. doi: 10.1016/j.ccell.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dorp L., Acman M., Richard D., et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maio M., Hamid O., Larkin J., et al. Immune-checkpoint inhibitors for cancer therapy in the COVID-19 era. Clin. Cancer Res. 2020;15:4201–4205. doi: 10.1158/1078-0432.CCR-20-1657. [DOI] [PubMed] [Google Scholar]
- 34.Di Giacomo A.M., Gambale E., Monterisi S., Valente M., Maio M. SARS-COV-2 infection in patients with cancer undergoing checkpoint blockade: clinical course and outcome. Eur. J. Cancer. 2020;133:1–3. doi: 10.1016/j.ejca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovly Cm, Boyd Kl, Gonzalez-Ericsson, et al. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv. 2020 doi: 10.1101/2020.04.29.20085738. [DOI] [Google Scholar]
- 36.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu R., Wu L., Zhang C., et al. Real-world scenario of patients with lung cancer amid the coronavirus disease 2019 pandemic in the People’s Republic of China. JTO Clinical and Research Reports. 2020:100053. doi: 10.1016/j.jtocrr.2020.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020;31:1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeffery M.M., D’Onofrio G., Paek H., et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern. Med. 2020:e203288. doi: 10.1001/jamainternmed.2020.3288. https://doi.org/10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database analysis. Gut. 2020 doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 41.de Joode K., Dumoulin D.W., Engelen V., et al. Impact of the coronavirus disease 2019 pandemic on cancer treatment: the patients’ perspective. Eur. J. Cancer. 2020;136:132–139. doi: 10.1016/j.ejca.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehanna H., Hardman J.C., Shenson J.A., et al. Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: an international consensus. Lancet Oncol. 2020;21:e350–e359. doi: 10.1016/S1470-2045(20)30334-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You B., Ravaud A., Canivet A., et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21:619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jheon S., Ahmed A.D., Fang V.W., et al. Thoracic cancer surgery during the COVID-19 pandemic: a consensus statement from the Thoracic Domain of the Asian Society for Cardiovascular and Thoracic Surgery. Asian Cardiovasc. Thorac. Ann. 2020;28:322–329. doi: 10.1177/0218492320940162. [DOI] [PubMed] [Google Scholar]
- 45.Pramesh C.S., Badwe R.A. Cancer management in India during Covid-19. N. Engl. J. Med. 2020;382:e61. doi: 10.1056/NEJMc2011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: Prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020;17:1–3. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spicer J., Chamberlain C., Papa S. Provision of cancer care during the COVID-19 pandemic. Nat. Rev. Clin. Oncol. 2020;17:329–331. doi: 10.1038/s41571-020-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curigliano G., Banerjee S., Cervantes A., et al. Managing cancer patients during the COVID-19 pandemic: an ESMO Interdisciplinary Expert Consensus. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Shamsi H.O., Alhazzani W., Alhuraiji A., et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of COVID-19. N. Engl. J. Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 51.Persad G., Wertheimer A., Emanuel E.J. Principles for allocation of scarce medical interventions. Lancet. 2009;373:423–431. doi: 10.1016/S0140-6736(09)60137-9. [DOI] [PubMed] [Google Scholar]
- 52.Marron J.M., Joffe S., Jagsi R., Spence R.A., Hlubocky F.J. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J. Clin. Oncol. 2020;38:2201–2205. doi: 10.1200/JCO.20.00960. [DOI] [PubMed] [Google Scholar]
- 53.White D.B., Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 54.Berlinger N., Wynia M., Powell T., et al. Ethical framework for health care institutions responding to novel coronavirus SARS-CoV-2 (COVID-19): guidelines for institutional ethics services responding to COVID-19. http://scha-files.s3.amazonaws.com/Documents/HastingsCenterCovidFramework2020.pdf.
- 55.Torzilli G., Vigano L., Galvanin J., et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann. Surg. 2020;272:e112–e117. doi: 10.1097/SLA.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thoracic Surgery Outcomes Research Network, et al. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. J. Thorac. Cardiovasc. Surg. 2020;160:601–605. doi: 10.1016/j.jtcvs.2020.03.061. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Leary M.P., Choong K.C., Thornblade L.W., Fakih M.G., Fong Y., Kaiser A.M. Management considerations for the surgical treatment of colorectal cancer during the global COVID-19 pandemic. Ann. Surg. 2020;272:e98–e105. doi: 10.1097/SLA.0000000000004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oba A., Stoop T.F., Lohr M., et al. Global survey on pancreatic surgery during the COVID-19 pandemic. Ann. Surg. 2020;272:e87–e93. doi: 10.1097/SLA.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thoracic Surgery Outcomes Research Network, et al. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. Ann. Thorac. Surg. 2020;110:692–696. doi: 10.1016/j.athoracsur.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barry A., Apisarnthanarax S., O’Kane G.M., et al. Management of primary hepatic malignancies during the COVID-19 pandemic: recommendations for risk mitigation from a multidisciplinary perspective. Lancet Gastroenterol. Hepatol. 2020;5:765–775. doi: 10.1016/S2468-1253(20)30182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallis C.J.D., Novara G., Marandino L., et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur. Urol. 2020;78:29–42. doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faivre-Finn C., Fenwick J.D., Franks K.N., et al. Reduced fractionation in lung cancer patients treated with curative-intent radiotherapy during the COVID-19 pandemic. Clin. Oncol. 2020;32:481–489. doi: 10.1016/j.clon.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monaghesh E., Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20:1193. doi: 10.21203/rs.3.rs-23906/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grenda T.R., Whang S., Evans N.R. Transitioning a surgery practice to telehealth during COVID-19. Ann. Surg. 2020;272:e168–e169. doi: 10.1097/SLA.0000000000004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163:104–106. doi: 10.1177/0194599820925049. [DOI] [PubMed] [Google Scholar]
- 66.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N. Engl. J. Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 67.Tan A.C., Ashley D.M., Khasraw M. Adapting to a pandemic – conducting oncology trials during the SARS-CoV-2 pandemic. Clin. Cancer Res. 2020;26:3100–3103. doi: 10.1158/1078-0432.CCR-20-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colbert L.E., Kouzy R., Abi Jaoude J., Ludmir E.B., Taniguchi C.M. Cancer research after COVID-19: where do we go from here? Cancer Cell. 2020;37:637–638. doi: 10.1016/j.ccell.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borno H.T., Small E.J. Does the COVID-19 outbreak identify a broader need for an urgent transformation of cancer clinical trials research? Contemp. Clin. Trials. 2020;92:105997. doi: 10.1016/j.cct.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unger J.M., Blanke C.D., LeBlanc M., Hershman Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10651. e2010651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waterhouse D.M., Harvey R.D., Hurley P., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol. Pract. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 72.London Jw, Fazio-Eynullayeva E., Palchuk Mb, Sankey P., McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin. Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yekeduz E., Karcioglu A.M., Utkan G., Urun Y. A clinical dilemma amid COVID-19 pandemic: Missed or encountered diagnosis of cancer? Future Oncol. 2020 doi: 10.2217/fon-2020-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zadnik V., Mihor A., Tomsic S., Zagar T., Bric N., Lokar K., Oblak I. Impact of COVID-19 on cancer diagnosis and management in Slovenia – preliminary results. Radiol. Oncol. 2020;54:329–334. doi: 10.2478/raon-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.https://baijiahao.baidu.com/s?id=1684493991705505333&wfr=spider&for=pc (accessed December 7, 2020).
- 76.https://www.thepaper.cn/newsDetail_forward_9974244 (accessed December 7, 2020).