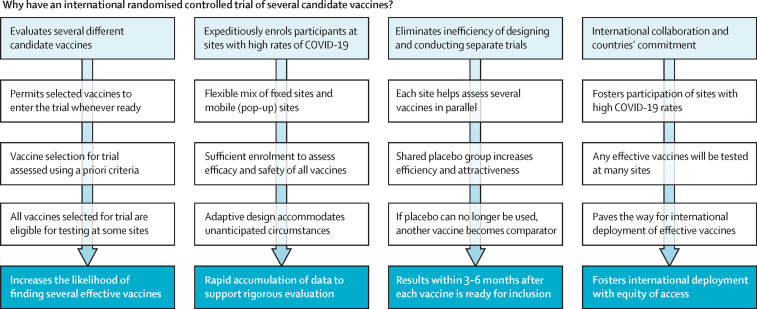
Figure.
Selected design features of the WHO Solidarity Vaccines Trial
The primary outcome is laboratory-confirmed symptoms >14 days after vaccination is completed. Analyses of each vaccine after about 40, 70, and 100 primary outcomes occur in the placebo group will report success if they show ≤10 versus 40, ≤30 versus 70, or ≤50 versus 100 outcomes. The third analysis is reported regardless of its findings. In all cases placebo-controlled follow-up continues until at least month 12 (or local deployment of an effective vaccine) to assess safety, disease severity, and duration of protection.