Highlights
-
•
Routine echocardiographic evaluation must be mandatory in patients with COVID-19 infection.
-
•
The presence of refractory hypoxemia and/or hypotension should raise the suspicion of right ventricle failure and must be evaluated with transthoracic echocardiogram.
-
•
In the clinical scenario of acute right ventricular failure, rising D-dimer and suspected pulmonary embolism, thrombolysis must be considered even without tomographic confirmation.
Keywords: COVID-19 infection, Acute heart failure, Echocardiography, Right ventricle, Thrombus
Abstract
Severe forms of COVID-19 infection are associated with the need for invasive mechanical ventilation and thromboembolic complications; those can affect the cardiac function especially the right ventricle performance. Critical care echocardiography has rapidly evolved as the election technique in the evaluation of the critically ill patients. This technique has the advantage that it can be done at patient´s bedside and helps to provide the appropriate treatment and to monitoring maneuver's response. We present 4 patients with a confirmed COVID-19 infection who presented with sudden hemodynamic and / or respiratory deterioration, in which transthoracic echocardiogram showed acute right ventricular failure as the trigger for the event and helped to guide an early therapeutic intervention.
<Learning objective: Routine echocardiographic evaluation must be mandatory in patients with COVID-19 infection. The presence of refractory hypoxemia and/or hypotension should raise the suspicion of right ventricle failure and must be evaluated with transthoracic echocardiogram. In the clinical scenario of acute right ventricular failure, rising D-dimer and suspected pulmonary embolism, thrombolysis must be considered even without tomographic confirmation.>
Introduction
Approximately, 20% of patients with COVID-19 infection will present with the severe form of the disease [1] and will require invasive mechanical ventilation to treat acute respiratory distress syndrome (ARDS). Additionally, some patients are at risk of developing thromboembolic complications [2].
Invasive mechanical ventilation and pulmonary embolism have adverse consequences in the heart-lung interaction, particularly in the right ventricular (RV) function [3] that can be evaluated with a transthoracic echocardiogram (TTE) [4].
The COVID-19 pandemic has highlighted the need for an adequate hemodynamic assessment to identify potential treatable causes that can modify prognosis. We present four cases of COVID-19 infection and acute right ventricular failure. Cases 1 and 2 represent the spectrum of pulmonary embolism and Case 3 and 4 represent acute cor pulmonale related with severe ARDS.
The following parameters were measured with TTE:
-
•
Right ventricular function: RV basal diameter, tricuspid annular plane systolic excursion (TAPSE), tricuspid peak systolic S wave tissue Doppler velocity (S' wave), fractional area change (FAC), RV basal diameter, RV/left ventricular (LV) ratio, pulmonary artery acceleration time (AcT), right atrial pressure, tricuspid regurgitation velocity (TRV), pulmonary artery systolic pressure (PASP), paradoxical septal movement, and the grade of tricuspid regurgitation.
-
•
Left ventricular function: left ventricular ejection fraction (LVEF) and the E/e’ ratio to estimate the filling pressure of the LV.
The echocardiographic views and parameters were recorded and measured according to the guidelines of the American Society of Echocardiography to perform a comprehensive transthoracic echocardiographic examination in adults, and the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging for cardiac chamber quantification [5,6].
Case 1
A 50-year-old male was admitted to the intensive care unit (ICU) following three days of odynophagia, headache, fever, and dyspnea. His vital signs showed tachypnea and hypoxemia that improved with supplementary oxygen by nasal cannula. The chest X-ray showed bilateral interstitial infiltrate. The laboratory findings were high levels of ferritin, C reactive protein, D-dimer, and troponin I with lymphopenia (Appendix Table 1). Twenty-four hours after admission the patient required invasive mechanical ventilation due to refractory hypoxemia. The arterial blood gases were compatible with severe ARDS; high inspired oxygen fraction (FiO2) levels and positive end-expiratory pressure (PEEP) titration were required; in the TTE the RV function was normal, defined by FAC ≥35%, TAPSE ≥17 mm, S wave ≥9.5 cm/s without dilatation of the RV [6] (Fig. 1A–C). Forty-eight hours after beginning invasive mechanical ventilation, he developed hypotension (with the need of norepinephrine 0.5 mcg/kg/min, vasopressin 0.06 U/min, and dobutamine 8 mcg/kg/min), sinus tachycardia, and worsening hypoxemia; electrocardiography (ECG) showed inverted T waves in V1–V4; a repeated TTE showed acute RV failure and a high probability of pulmonary hypertension defined by 1) TRV of 2.9–3.4 m/s in the presence of 2 of the following: RV/LV ratio >1.0 and/or paradoxical septal movement, AcT <105 msec, and inferior vena cava diameter >21 mm with decreased inspiratory collapse <50% (during positive pressure ventilation, the inspiratory change is not parallel with right atrial pressure) or 2) TRV >3.4 m/s [7], in this patient their findings were TRV 3.4 m/s, RV/LV ratio 1.2, and paradoxical septal movement; LVEF was slightly reduced (50%) without elevation of the E/e’ ratio (Table 1, Fig. 1D–F). Due to the presence of acute RV failure associated with hypotension and elevated D-dimer, in this context with high suspicion of pulmonary embolism and associated hemodynamic instability, thrombolysis with alteplase 100 mg was administered without evidence of bleeding, with improvement of the RV function. The refractory hypoxemia could not be reverted and the patient died.
Fig. 1.
Transthoracic echocardiogram (TTE) of case 1 (24 h after invasive mechanical ventilation). (A) Apical 4-chamber view without right ventricular (RV) dilatation. (B) TAPSE (measured by aligning an M-mode cursor parallel with the RV free wall as it meets the tricuspid annulus from the RV apical four-chamber view) with a normal value (17.8 mm). (C) S wave (measured with tissue Doppler placing the sample volume at the tricuspid annular level) with a normal value (13.6 cm/s). TTE ofcase 1after hemodynamic deterioration. (D) Dilated RV with a RV/LV ratio >1. (E) Diminished TAPSE (12 mm). (F) Diminished S wave (9.4 cm/s); both compatible with longitudinal RV systolic dysfunction.
TAPSE, tricuspid annular plane systolic excursion; S wave, tricuspid peak systolic S wave tissue Doppler velocity; LV, left ventricle; RA, right atrium; LA, left atrium.
Table 1.
Echocardiographic findings.
| Case | TAPSE | S’ Wave | FAC | RV basal diameter | RV/LV ratio | AcT | TRV | RAP | PASP | PSM* | TR | LVEF | E/e'ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.2 | 9.41 | 33 | 45 | 1,2 | 60 | 3.4 | 5 | 51 | Yes | M | 50 | 7 |
| 2 | 11 | 4 | 23 | 51 | 1.3 | 62 | 2.7 | 5 | 34 | Yes | M | 52 | 10 |
| 3 | 20 | 9.98 | 25 | 50 | 1.3 | 44 | 3.2 | 10 | 51 | Yes | NA | 40 | 8 |
| 4 | 21 | 13 | 40 | 45 | 1.1 | 45 | 3.6 | 10 | 62 | Yes | M | 51 | 11 |
EF, ejection fraction (%); FAC, fractional area change (%); LV, left ventricle; PASP, pulmonary artery systolic pressure (mmHg); AcT, pulmonary artery acceleration time (msec); PSM, paradoxical septal movement; RAP, right atrial pressure (mmHg); RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion (mm); S wave, tricuspid peak systolic S wave tissue Doppler velocity (cm/s); TR, tricuspid regurgitation severity (M: moderate, S: severe); TRV, tricuspid regurgitation velocity (m/s); NA, not available.
Normal values: TAPSE ≥17 mm, S wave ≥9.5 cm/s, FAC ≥35%, RV basal diameter <41 mm, RV/LV ratio <1, AcT >105 mseg, LVEF >52% in men, >54% in woman, E/e’ ratio <14.
Qualitative.
Case 2
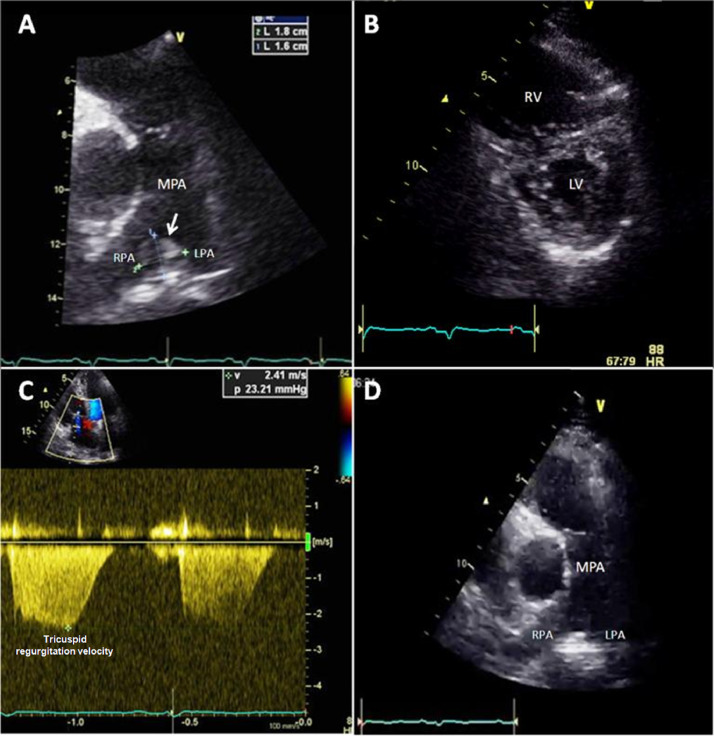
A 55-year-old male with past history of smoking and type 2 diabetes was admitted to ICU following seven days of cough and in the previous two days dyspnea and chest pain. His vital signs showed tachycardia, tachypnea, and hypoxemia that improved with supplementary oxygen by nasal cannula. Chest X-ray showed bilateral interstitial infiltrates. The laboratory findings were high levels of D-dimer, troponin I, and N-terminal pro-brain natriuretic peptide (NT-proBNP) (Appendix Table 1). Twelve-lead ECG showed sinus tachycardia and McGinn-White sign (S1Q3T3). Pulmonary embolism was suspected; the TTE showed a thrombus at the bifurcation of the main pulmonary artery (Fig. 2A, Video 1), RV dilatation (Fig. 2B), and systolic dysfunction¸ LVEF and the E/e’ ratio were in the normal range (Table 1). The computed tomography pulmonary angiogram confirmed the diagnosis of high-risk pulmonary embolism because of the presence of hypotension (with the need for norepinephrine 0.1 mcg/kg/min and dobutamine 3 mcg/kg/min) and elevated biomarkers (NT-proBNP and troponin I), so thrombolysis with alteplase 100 mg was administered without evidence of bleeding, with improvement in the RV function and disappearance of the thrombus (Fig. 2D). The patient was discharged from ICU 48 h after thrombolysis without symptoms and with reduction in oxygen requirements.
Fig. 2.
Transthoracic echocardiogram (TTE) of case 2. (A) 1.8 × 1.6 cm thrombus at the pulmonary artery bifurcation (arrow). (B) Parasternal short-axis view at mid-ventricular level showing D-shaped left ventricle (LV) in systole suggesting right ventricular (RV) pressure overload. (C) Tricuspid regurgitation velocity of 2.4 m/s; in the context of a shortened acceleration time (62 msec) suggested that there was acute pressure overload of the RV. (D) Disappearance of the thrombus after thrombolysis was demonstrated.
MPA, main pulmonary artery; RPA, right pulmonary artery; LPA, left pulmonary artery.
Case 3
A 67-year-old female with past history of type 2 diabetes, hypertension, and obesity was admitted to ICU following 14 days of fever, dry cough, and dyspnea in the previous two days. Her vital signs showed tachypnea, tachycardia, and hypoxemia. The condition improved with supplementary oxygen by nasal cannula. Chest X-ray showed bilateral interstitial infiltrates. Initial laboratory findings were elevated ferritine and C-reactive protein (Appendix Table 1). Twenty-four hours after admission she developed moderate ARDS and invasive mechanical ventilation was initiated; in the TTE RV function was normal at that time. Eleven days after the initiation of invasive mechanical ventilation, the patient developed hypotension, hypoxemia, and acute renal failure. The ECG showed incomplete right bundle branch block; a repeated TTE showed high probability of pulmonary hypertension and RV dilatation with systolic dysfunction; LVEF was moderately reduced (40%) without elevation of the E/e’ ratio (Table 1). Levosimendan 0.15 mcg/kg/min was initiated, with hemodynamic improvement. Seventy-two hours after, the patient developed refractory septic shock (requirements for norepinephrine up to 0.6 mcg/kg/min and vasopressin 0.06 U/min) and died.
Case 4
A 56-year-old male with type 2 diabetes was admitted to the ICU following three days of fever, dyspnea, and cough. His vital signs showed tachypnea and hypoxemia that improved with non-rebreathing oxygen mask. Chest X-ray showed bilateral interstitial infiltrates. Laboratory findings were high levels of C reactive protein, D-dimer, troponin I, and ferritin (Appendix Table 1). Six days after admission the patient required invasive mechanical ventilation due to severe ARDS (PaO2/FIO2 < 100). Forty-eight hours after, the patient presented hemodynamic collapse so vasopressors and inotropes were required (norepinephrine up to 0.8 mcg/kg/min, dobutamine 6 mcg/kg/min, and levosimendan 0.2 mcg/kg/min). ECG showed tall R waves and ST segment and T wave inversion in V1–V2. The TTE showed RV dilatation and systolic dysfunction with high probability of pulmonary hypertension¸ LVEF was slightly reduced (51%) without elevation of the E/e’ ratio (Table 1). He developed cardiogenic shock due to refractory right ventricular failure and died 24 h later.
Discussion
We present 4 cases of acute RV failure in patients with COVID-19 infection, which was identified due to systematic echocardiographic evaluation.
Refractory hypoxemia, high FiO2, and PEEP used during invasive mechanical ventilation have deleterious effects on the RV performance such as increase in intrathoracic pressure, pulmonary vascular resistance, RV afterload, and decrease in RV compliance, which can cause hemodynamic collapse [3,8].
Additionally, patients with COVID-19 infection are at high risk of thromboembolic disease [2], which could worsen RV function [9]. Current guidelines suggest that in patients with hemodynamic instability, TTE facilitates the decision to treat patients with systemic thrombolysis. In two of our four cases, systemic thrombolysis was administered and after this treatment the patients showed clinical improvement.
The TTE in critical ill patients is the cornerstone of the hemodynamic evaluation [5]. This tool allows real-time assessment of the patient, to reach the diagnosis, and to perform therapeutic maneuvers that can modify the prognosis of the disease. With the COVID-19 pandemic, the priority is to minimize the exposure of health personnel, without affecting the quality of care. In our case series, the use of TTE allowed the correct diagnosis of the hemodynamic profile and to monitor treatment. All studies were performed during regular patient rounds, the machine and sector probe were sanitized between each evaluation, all providers had adequate personal protective equipment, images were first recorded, and all measurements were performed outside the patient's room. A recently published critical care ultrasonography algorithm described the feasibility to perform the evaluation in 18 min, without additional risk of contagion. This tool has the flexibility to be extended if any pathological finding is identified, such as the case of RV dysfunction. Adding the TRV and the quantification of the AcT would not take much additional time and would provide highly relevant clinical information [10].
Critically ill patients with COVID-19 infection should have hemodynamic monitoring based on serial ultrasound, with a focus on RV function, because acute RV failure has a high morbidity. This strategy is not only safe, but also allows prompt diagnosis of potentially treatable conditions such as pulmonary embolism; systemic thrombolysis should be administered in selected cases with hemodynamic deterioration. The application of local algorithms based on TTE should be applied during the COVID-19 pandemic.
Declaration of Competing Interest
No conflicts of interest to disclose.
Acknowledgments
To the INC Critical Care Ultrasonography Working Group.
Footnotes
Funding sources: None.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.01.001.
Appendix. Supplementary materials
References
- 1.Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood S, Pinsky M. Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med. 2018;6(1-8):e349. doi: 10.21037/atm.2018.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan S, Schmidt G. Acute right ventricular dysfunction. Chest. 2015;147:835–846. doi: 10.1378/chest.14-1335. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. [Google Scholar]
- 8.Creel-Bulos C, Hockstein M, Amin N, Melhem S, Truong A, Sharifpour M. Acute cor pulmonale in critically ill patients with Covid-19. NEJM. 2020;382(1-4):e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah W, Saeed R, Sarwar U, Patel R, Fischman D. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020;2:1379–1382. doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Cruz E, Manzur-Sandoval D, Rascón-Sabido R, Gopar-Nieto R, Barajas-Campos RL, Jordán-Ríos A, Sierra-Lara Martínez D, Jiménez-Rodríguez GM, Murillo-Ochoa AL, Díaz-Méndez A, Lazcano-Díaz E, Araiza-Garaygordobil D, Cabello-López A, Melano-Carranza E, Bucio-Reta E. Critical care ultrasonography during COVID-19 pandemic: the ORACLE protocol. Echocardiography. 2020;37:1353–1361. doi: 10.1111/echo.14837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.