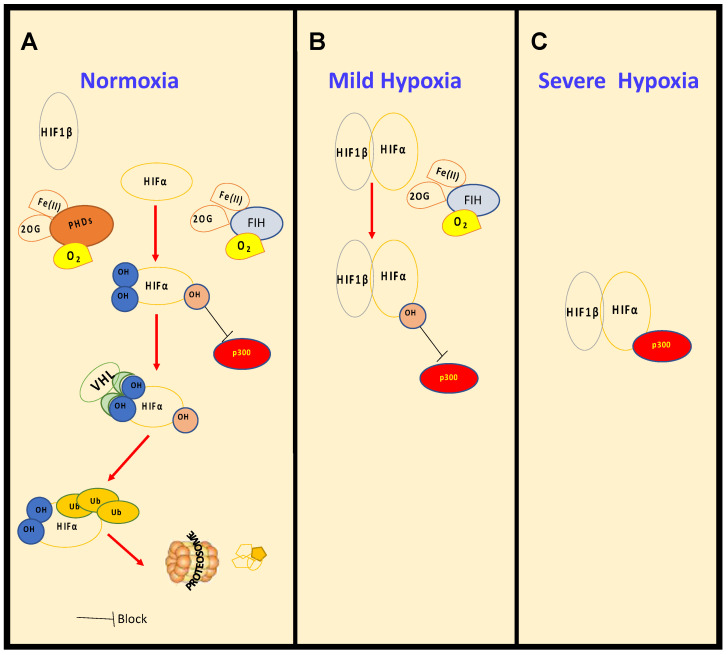
Figure 1.
Regulation of HIF levels and activity in normoxia and hypoxia. Under normal oxygen conditions, (A), normoxia, HIF-α is constantly hydroxylated by PHDs and FIH. PHD-mediated hydroxylation increases binding affinity with the tumour suppressor VHL, which promotes ubiquitination and degradation by the proteosome. As oxygen levels decrease, in mild hypoxia (15–1% O2) (B), PHDs are inhibited, HIF-α is stabilised, though still hydroxylated by FIH, binds to HIF-1β and is able to induce transcription of certain target genes. With further reduction in oxygen levels, in severe hypoxia (<1% O2) (C), FIH is also inhibited and HIF is able to become fully active by the recruitment of co-activators such as p300.