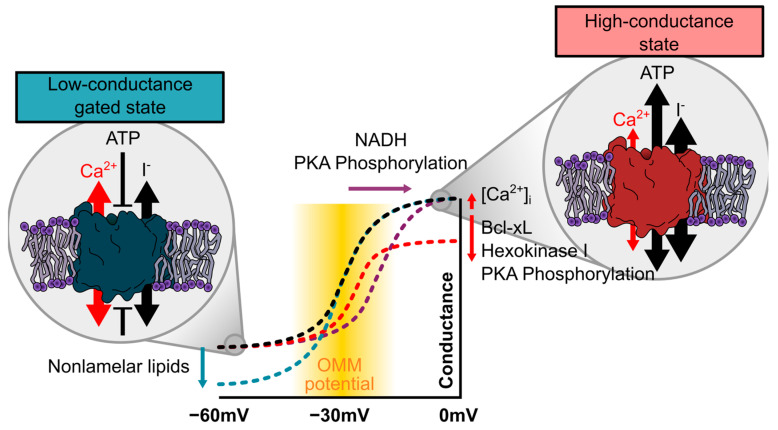
Figure 2.
Regulation of voltage-dependent gating of VDAC. While VDAC almost exclusively resides in its anion-selective and metabolite-permeable high-conductance state at neutral potentials, it starts gating within the range of −20 to −30 mV (black line), which is well within the predicted range of an OMM potential (yellow zone). Note that the conductance–voltage relation of VDAC is symmetrical, and only the negative side is depicted. At potentials below −40 mV, it almost exclusively resides in the low-conductance state that is impermeable for ATP but shows a higher permeability of Ca2+. Several factors were reported to modulate this voltage–conductance relationship. The phosphorylation status or binding of protein partners such as Bcl-xL or hexokinase were suggested to facilitate channel gating and, thus, induce both a right-shift of the voltage dependence and a reduction in the maximum conductance at potentials close to neutral (red line), while Ca2+ is suggested to facilitate channel opening and, thus, has an opposite effect. Nonlamellar lipids were demonstrated to promote channel closure at very negative potentials (blue).