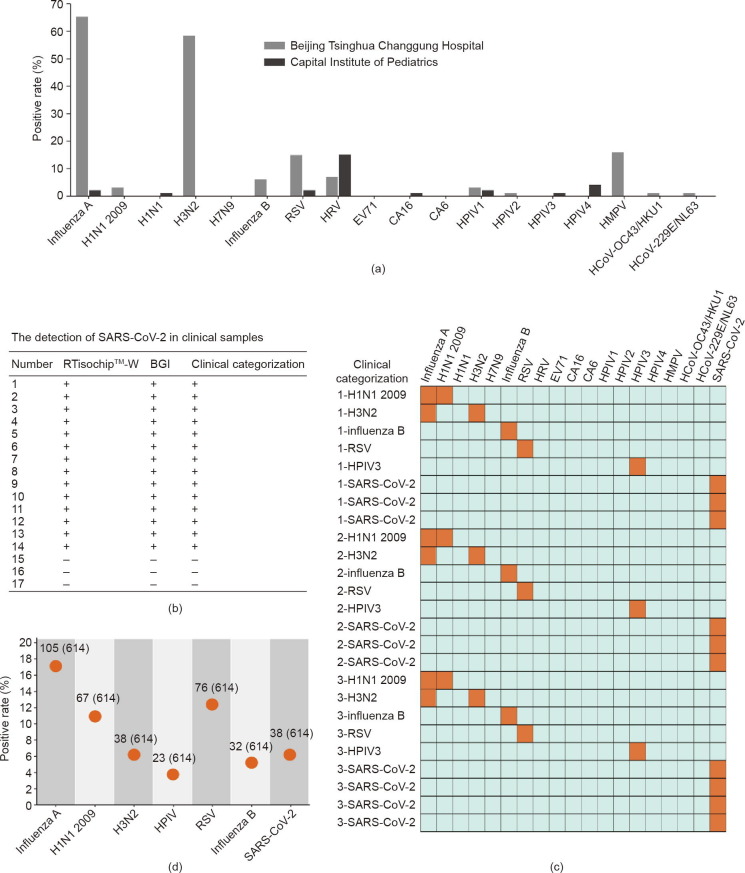
Fig. 6.
Clinical identification of respiratory viruses using the RTisochip™-W. (a) Positive rates of each virus target in clinical samples collected in 2019–2020. Samples from the Beijing Tsinghua Changgung Hospital were collected in the flu season from 2019 to 2020 (n = 101), and samples from the Capital Institute of Pediatrics were collected in the non-flu season of 2019 (n = 100). (b) Comparison of the SARS-CoV-2 detection results obtained by the RTisochip™-W and the conventional RT-PCR. “+” represents a positive result and “–” is negative. (c) Repeatability tests of the RTisochip™-W using clinical samples. Positive and negative results are shown as orange and azure squares, respectively. (d) Positive rates of a total of 614 clinical throat swab samples from suspected or confirmed COVID-19 patients. The detailed number was indicated.