Figure 4.
Cryo-EM Structure of the Nsp1-40S Ribosome Complex
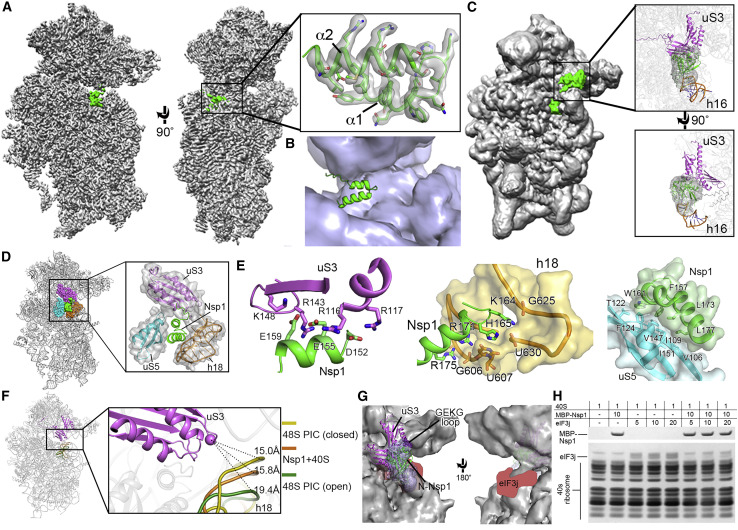
(A) Overall density of the Nsp1-40S ribosome complex with Nsp1 (green) and 40S ribosome (gray). Inset shows C-Nsp1 with corresponding density with clear sidechain features. C-Nsp1 α helices (α1, aa 154–160; α2, aa 166–179) are labeled.
(B) Cross section of the C-Nsp1 (green) within the mRNA entry channel. 40S ribosome is shown in surface, and C-Nsp1 is displayed in cartoon.
(C) Overall density of Nsp1-40S ribosome complex at a lower contour level. Insets show the extra globular density with SARS-CoV Nsp1 N-terminal domain (PDB: 2HSX, green) fitted. Ribosomal protein uS3 (magenta) and rRNA h16 (orange) are shown in cartoon.
(D) Overall structure of the C-Nsp1-40S ribosome complex, with C-Nsp1 (green surface) and the surrounding protein uS3 (magenta sphere representation), uS5 (cyan) and rRNA h18 (orange) highlighted. The inset shows zoomed-in view of C-Nsp1 in cartoon, with the surrounding 40S components in cartoon and surface to illustrate the mRNA entry channel.
(E) Molecular interactions between C-Nsp1 and 40S ribosome components, including uS3, h18, and uS5. Proteins and rRNA are in the same color as in (D) and shown in cartoon, with binding pocket and hydrophobic interface depicted in surface. The interacting residues are shown in sticks.
(F) The conformation of the 40S ribosome in the Nsp1-40S complex is similar to the close form in the 48S PIC. Q179 of uS3 (magenta cartoon) is displayed as a sphere. h18 is in cartoon and colored dark yellow (48S closed conformation), orange (Nsp1-40S ribosome complex), and dark green (48S open conformation), with distances to Q179 indicated by the dashes.
(G) The N-terminal domain of Nsp1 covers uS3 surface on the solvent side. The cryo-EM density in this region is shown in blue surface with SARS-CoV Nsp1 N-terminal domain (PDB: 2HSX) fitted. uS3 (magenta) is depicted in cartoon. The GEKG loop (dark purple) is shown in sphere representation. The putative location of eIF3j is marked in red.
(H) SDS-PAGE analysis of Nsp1 and eIF3j competition at different concentration ratios (indicated in the top table).
See also Figures S3–S5.