Figure 5.
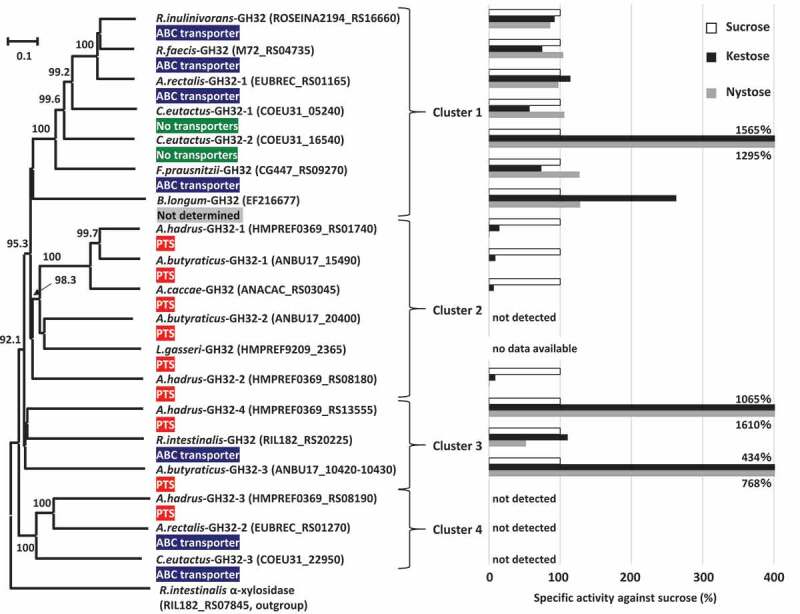
Phylogenetic relationships of GH32 enzymes found in butyrate-producing bacteria, and their relative specific activities with kestose and nystose against sucrose. Locus tags of each protein are shown in parenthesis and transporters adjacent to each GH32 protein are supplied. Extracellular GH32 enzymes (A.hadrus-GH32-4 and A.butyraticus-GH32-3) were expressed using a surface display system on E. coli cells, and whole cells of the transformed E. coli were included to assess the activities of the GH32 enzymes on sucrose, kestose, and nystose. For intracellular GH32 enzymes, cell-free extracts prepared from the transformed E. coli strains were used to assess the activities. Relative specific activities (%) of recombinant GH32 enzymes with kestose and nystose against sucrose are shown. For A.butyraticus-GH32-3, specific activities by the fused enzyme (ANBU17_10420 and ANBU17_10430) are shown, and those by the partial A.butyraticus-GH32-3 (ANBU17_10420) were unable to be assessed because of the lack of activity with sucrose. B.longum-GH32 and L.gasseri-GH32, originating from B. longum KN29.1 and L. gasseri 224–1, respectively, were included as reference proteins, and a possible transporter for B.longum-GH32 was unable to be identified due to the unavailability of genome data for B. longum KN29.1. Specific activities for B.longum-GH32 and A.caccae-GH32 were obtained from previous reports.32,33 α-Xylosidase in Roseburia intestinalis (RIL182_RS07845) was used as an outgroup. Bootstrap percentages above 70% are given at branching points
