Figure 2.
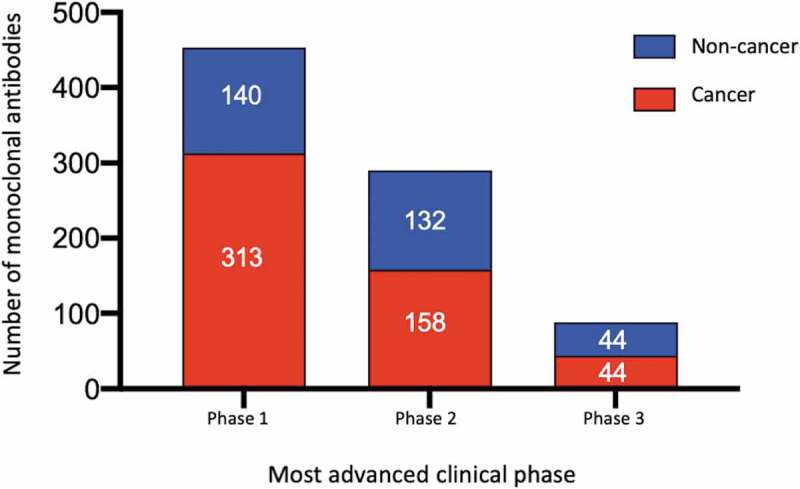
Global commercial clinical pipeline of monoclonal antibodies*
*Data publicly available as of November 15, 2020. Red bars, most advanced clinical study is in cancer. Blue bars, most advanced clinical study is in non-cancer indications. Approved or authorized mAb products, investigational mAbs in regulatory review, and all biosimilar antibodies and Fc fusion proteins were excluded. Phase 1/2 studies were included with Phase 2; pivotal or registrational Phase 2 and Phase 2/3 studies were included with Phase 3.
